当前位置:
X-MOL 学术
›
ChemSusChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Capsule-Structured Copper-Zinc Catalyst for Highly Efficient Hydrogenation of Carbon Dioxide to Methanol.
ChemSusChem ( IF 7.5 ) Pub Date : 2019-10-22 , DOI: 10.1002/cssc.201902485 Yongle Guo 1 , Xinwen Guo 1 , Chunshan Song 1, 2 , Xinghua Han 3 , Hongyang Liu 4 , Zhongkui Zhao 1
ChemSusChem ( IF 7.5 ) Pub Date : 2019-10-22 , DOI: 10.1002/cssc.201902485 Yongle Guo 1 , Xinwen Guo 1 , Chunshan Song 1, 2 , Xinghua Han 3 , Hongyang Liu 4 , Zhongkui Zhao 1
Affiliation
|
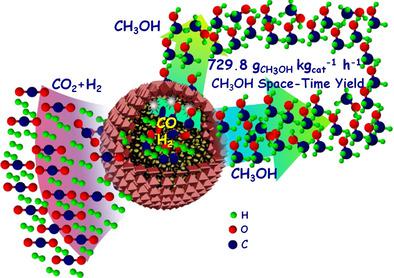
To develop a new and efficient CO2 -to-methanol catalyst is of extreme significance but still remains a challenge. Herein, an innovative indirect two-step strategy is reported to synthesize a highly efficient capsule-structured copper-based CO2 -to-methanol catalyst (CZA-r@CZM). It consists of a structurally reconstructed millimeter-sized Cu/ZnO/Al2 O3 core (CZA-r) with intensified Cu-ZnO interactions, which is made by a facile hydrothermal treatment in an alkaline aqueous solution, and a Cu/ZnO/MgO (CZM) shell prepared by an ethylene glycol-assisted physical coating method. The CZA-r core displays 2.7 times higher CO2 hydrogenation activity with 2.0 times higher CO selectivity than the previously reported Cu/ZnO/Al2 O3 (CZA-p), whereas the CZM shell can efficiently catalyze hydrogenation of the as-formed CO from the CZA-r core to methanol as it passes through the shell. As a result, the developed capsule-structured CZA-r@CZM catalyst exhibits 2.4 times higher CO2 conversion with 1.8 times higher turnover frequency and 2.3-fold higher methanol space-time yield than the CZA-p catalyst (729.8 vs. 312.6 gMeOH kgcat -1 h-1 ). In situ diffuse reflectance infrared Fourier transform spectroscopy (DRIFTs) experiments reveal that the CO2 hydrogenation reaction proceeds through a reverse water-gas shift reaction followed by a CO hydrogenation pathway via an *H3 CO intermediate. This work not only produces an efficient CO2 -to-methanol catalyst, but also opens a new avenue for designing superior catalysts for other consecutive transformations.
中文翻译:

胶囊结构的铜锌催化剂,用于将二氧化碳高效加氢为甲醇。
开发一种新型高效的二氧化碳转化为甲醇的催化剂具有极其重要的意义,但仍然是一个挑战。在此,据报道,创新的间接两步策略可合成高效的胶囊结构的铜基CO2转化为甲醇的催化剂(CZA-r @ CZM)。它由结构重整的毫米大小的Cu / ZnO / Al2 O3核(CZA-r)和Cu / ZnO / MgO构成,该核通过增强的Cu-ZnO相互作用(在碱性水溶液中进行便捷的水热处理)制成。 CZM)壳,通过乙二醇辅助物理涂覆方法制备。与以前报道的Cu / ZnO / Al2 O3(CZA-p)相比,CZA-r核显示出高出2.7倍的CO2加氢活性和2.0倍的CO选择性,而CZM壳可以有效地催化形成的一氧化碳从CZA-r核穿过壳时的加氢反应。结果,与CZA-p催化剂相比,开发的胶囊结构CZA-r @ CZM催化剂的CO2转化率高2.4倍,周转频率高1.8倍,甲醇时空产率高2.3倍(729.8 vs. 312.6 gMeOH kgcat -1 h-1)。原位漫反射红外傅里叶变换光谱(DRIFTs)实验表明,CO2加氢反应通过反向水煤气变换反应进行,然后通过* H3 CO中间体进行CO加氢途径。这项工作不仅产生了一种高效的二氧化碳转化为甲醇的催化剂,而且为设计用于其他连续转化的优良催化剂开辟了一条新途径。与CZA-p催化剂相比,开发的胶囊结构CZA-r @ CZM催化剂的CO2转化率高2.4倍,周转频率高1.8倍,甲醇时空产率高2.3倍(729.8 vs. 312.6 gMeOH kgcat -1 h- 1)。原位漫反射红外傅里叶变换光谱(DRIFTs)实验表明,CO2加氢反应通过反向水煤气变换反应进行,然后通过* H3 CO中间体进行CO加氢途径。这项工作不仅产生了一种高效的二氧化碳转化为甲醇的催化剂,而且为设计用于其他连续转化的优良催化剂开辟了一条新途径。与CZA-p催化剂相比,开发的胶囊结构CZA-r @ CZM催化剂的CO2转化率高2.4倍,周转频率高1.8倍,甲醇时空产率高2.3倍(729.8 vs. 312.6 gMeOH kgcat -1 h- 1)。原位漫反射红外傅里叶变换光谱(DRIFTs)实验表明,CO2加氢反应通过反向水煤气变换反应进行,然后通过* H3 CO中间体进行CO加氢途径。这项工作不仅产生了一种高效的二氧化碳转化为甲醇的催化剂,而且为设计用于其他连续转化的优良催化剂开辟了一条新途径。甲醇时空产率比CZA-p催化剂高3倍(729.8对312.6 gMeOH kgcat -1 h-1)。原位漫反射红外傅里叶变换光谱(DRIFTs)实验表明,CO2加氢反应通过反向水煤气变换反应进行,然后通过* H3 CO中间体进行CO加氢途径。这项工作不仅产生了一种高效的二氧化碳转化为甲醇的催化剂,而且为设计用于其他连续转化的优良催化剂开辟了一条新途径。甲醇时空产率比CZA-p催化剂高3倍(729.8对312.6 gMeOH kgcat -1 h-1)。原位漫反射红外傅里叶变换光谱(DRIFTs)实验表明,CO2加氢反应通过反向水煤气变换反应进行,然后通过* H3 CO中间体进行CO加氢途径。这项工作不仅产生了一种高效的二氧化碳转化为甲醇的催化剂,而且为设计用于其他连续转化的优良催化剂开辟了一条新途径。
更新日期:2019-10-23
中文翻译:

胶囊结构的铜锌催化剂,用于将二氧化碳高效加氢为甲醇。
开发一种新型高效的二氧化碳转化为甲醇的催化剂具有极其重要的意义,但仍然是一个挑战。在此,据报道,创新的间接两步策略可合成高效的胶囊结构的铜基CO2转化为甲醇的催化剂(CZA-r @ CZM)。它由结构重整的毫米大小的Cu / ZnO / Al2 O3核(CZA-r)和Cu / ZnO / MgO构成,该核通过增强的Cu-ZnO相互作用(在碱性水溶液中进行便捷的水热处理)制成。 CZM)壳,通过乙二醇辅助物理涂覆方法制备。与以前报道的Cu / ZnO / Al2 O3(CZA-p)相比,CZA-r核显示出高出2.7倍的CO2加氢活性和2.0倍的CO选择性,而CZM壳可以有效地催化形成的一氧化碳从CZA-r核穿过壳时的加氢反应。结果,与CZA-p催化剂相比,开发的胶囊结构CZA-r @ CZM催化剂的CO2转化率高2.4倍,周转频率高1.8倍,甲醇时空产率高2.3倍(729.8 vs. 312.6 gMeOH kgcat -1 h-1)。原位漫反射红外傅里叶变换光谱(DRIFTs)实验表明,CO2加氢反应通过反向水煤气变换反应进行,然后通过* H3 CO中间体进行CO加氢途径。这项工作不仅产生了一种高效的二氧化碳转化为甲醇的催化剂,而且为设计用于其他连续转化的优良催化剂开辟了一条新途径。与CZA-p催化剂相比,开发的胶囊结构CZA-r @ CZM催化剂的CO2转化率高2.4倍,周转频率高1.8倍,甲醇时空产率高2.3倍(729.8 vs. 312.6 gMeOH kgcat -1 h- 1)。原位漫反射红外傅里叶变换光谱(DRIFTs)实验表明,CO2加氢反应通过反向水煤气变换反应进行,然后通过* H3 CO中间体进行CO加氢途径。这项工作不仅产生了一种高效的二氧化碳转化为甲醇的催化剂,而且为设计用于其他连续转化的优良催化剂开辟了一条新途径。与CZA-p催化剂相比,开发的胶囊结构CZA-r @ CZM催化剂的CO2转化率高2.4倍,周转频率高1.8倍,甲醇时空产率高2.3倍(729.8 vs. 312.6 gMeOH kgcat -1 h- 1)。原位漫反射红外傅里叶变换光谱(DRIFTs)实验表明,CO2加氢反应通过反向水煤气变换反应进行,然后通过* H3 CO中间体进行CO加氢途径。这项工作不仅产生了一种高效的二氧化碳转化为甲醇的催化剂,而且为设计用于其他连续转化的优良催化剂开辟了一条新途径。甲醇时空产率比CZA-p催化剂高3倍(729.8对312.6 gMeOH kgcat -1 h-1)。原位漫反射红外傅里叶变换光谱(DRIFTs)实验表明,CO2加氢反应通过反向水煤气变换反应进行,然后通过* H3 CO中间体进行CO加氢途径。这项工作不仅产生了一种高效的二氧化碳转化为甲醇的催化剂,而且为设计用于其他连续转化的优良催化剂开辟了一条新途径。甲醇时空产率比CZA-p催化剂高3倍(729.8对312.6 gMeOH kgcat -1 h-1)。原位漫反射红外傅里叶变换光谱(DRIFTs)实验表明,CO2加氢反应通过反向水煤气变换反应进行,然后通过* H3 CO中间体进行CO加氢途径。这项工作不仅产生了一种高效的二氧化碳转化为甲醇的催化剂,而且为设计用于其他连续转化的优良催化剂开辟了一条新途径。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号