当前位置:
X-MOL 学术
›
Chem. Bio. Drug Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
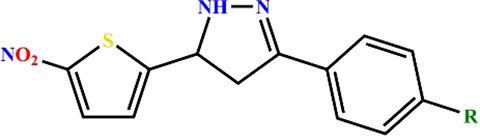
Exploration of 5-(5-nitrothiophen-2-yl)-4,5-dihydro-1H-pyrazoles as selective, multitargeted antimycobacterial agents.
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2019-10-14 , DOI: 10.1111/cbdd.13624 Neha Agre 1, 2 , Mihir Khambete 1 , Arundhati Maitra 2 , Antima Gupta 2 , Tulika Munshi 3 , Sanjib Bhakta 2 , Mariam Degani 1
Chemical Biology & Drug Design ( IF 3.2 ) Pub Date : 2019-10-14 , DOI: 10.1111/cbdd.13624 Neha Agre 1, 2 , Mihir Khambete 1 , Arundhati Maitra 2 , Antima Gupta 2 , Tulika Munshi 3 , Sanjib Bhakta 2 , Mariam Degani 1
Affiliation
|
We report the biological evaluation of 5-(5-nitrothiophen-2-yl)-4,5-dihydro-1H-pyrazole derivatives against bacteria, eukaryotic cell lines and the assessment of their mechanisms of action to determine their prospects of being developed into potent antituberculosis agents. The compounds were evaluated for their antibacterial property against Mycobacterium tuberculosis H37Rv, multidrug-resistant M. tuberculosis, Mycobacterium bovis BCG, Mycobacterium aurum, Escherichia coli, and Staphylococcus aureus using high-throughput spot-culture growth inhibition assay. They were found to be selective toward slow-growing mycobacteria and Gram-positive bacteria. In M. bovis BCG, they exhibited a bactericidal mode of action. Cytotoxicity was assessed in human THP-1 and murine RAW 264.7 cell lines, and the compounds showed a lower cytotoxicity potential when compared with their antibacterial activity. They were found to be excellent whole-cell efflux pump inhibitors of the mycobacterial surrogate M. aurum, performing better than known efflux pump inhibitor verapamil. The 5-nitrothiophene moiety was identified for the first time as a prospective inhibitor scaffold of mycobacterial arylamine N-acetyltransferase enzyme, which is the key enzyme in metabolizing isoniazid, a first-line antituberculosis drug. The two aforementioned findings make the compounds potential hits in the development of adjunctive tuberculosis therapy.
中文翻译:

探索5-(5-硝基噻吩-2-基)-4,5-二氢-1H-吡唑类化合物作为多目标抗分枝杆菌的选择性药物。
我们报告了对细菌,真核细胞系的5-(5-硝基噻吩-2-基)-4,5-二氢-1H-吡唑衍生物的生物学评估,以及对其作用机理的评估,以确定它们的发展前景。强效抗结核药。使用高通量斑点培养生长抑制测定法评估了这些化合物对结核分枝杆菌H37Rv,耐多药结核分枝杆菌,牛分枝杆菌BCG,金黄色分枝杆菌,大肠杆菌和金黄色葡萄球菌的抗菌性能。发现它们对缓慢生长的分枝杆菌和革兰氏阳性细菌具有选择性。在牛分枝杆菌BCG中,它们表现出杀菌作用模式。在人类THP-1和鼠RAW 264.7细胞系中评估了细胞毒性,与它们的抗菌活性相比,这些化合物显示出较低的细胞毒性潜力。发现它们是分枝杆菌替代金黄色葡萄球菌的优秀全细胞外排泵抑制剂,其性能优于已知的外排泵抑制剂维拉帕米。5-硝基噻吩部分首次被鉴定为分枝杆菌芳胺N-乙酰基转移酶的前瞻性抑制剂支架,这是代谢一线抗结核药物异烟肼的关键酶。上述两个发现使该化合物在辅助结核治疗的发展中具有潜在的优势。5-硝基噻吩部分首次被鉴定为分枝杆菌芳胺N-乙酰基转移酶的前瞻性抑制剂支架,这是代谢一线抗结核药物异烟肼的关键酶。上述两个发现使该化合物在辅助结核治疗的发展中具有潜在的优势。5-硝基噻吩部分首次被鉴定为分枝杆菌芳胺N-乙酰基转移酶的前瞻性抑制剂支架,这是代谢一线抗结核药物异烟肼的关键酶。上述两个发现使该化合物在辅助结核治疗的发展中具有潜在的优势。
更新日期:2019-10-14
中文翻译:

探索5-(5-硝基噻吩-2-基)-4,5-二氢-1H-吡唑类化合物作为多目标抗分枝杆菌的选择性药物。
我们报告了对细菌,真核细胞系的5-(5-硝基噻吩-2-基)-4,5-二氢-1H-吡唑衍生物的生物学评估,以及对其作用机理的评估,以确定它们的发展前景。强效抗结核药。使用高通量斑点培养生长抑制测定法评估了这些化合物对结核分枝杆菌H37Rv,耐多药结核分枝杆菌,牛分枝杆菌BCG,金黄色分枝杆菌,大肠杆菌和金黄色葡萄球菌的抗菌性能。发现它们对缓慢生长的分枝杆菌和革兰氏阳性细菌具有选择性。在牛分枝杆菌BCG中,它们表现出杀菌作用模式。在人类THP-1和鼠RAW 264.7细胞系中评估了细胞毒性,与它们的抗菌活性相比,这些化合物显示出较低的细胞毒性潜力。发现它们是分枝杆菌替代金黄色葡萄球菌的优秀全细胞外排泵抑制剂,其性能优于已知的外排泵抑制剂维拉帕米。5-硝基噻吩部分首次被鉴定为分枝杆菌芳胺N-乙酰基转移酶的前瞻性抑制剂支架,这是代谢一线抗结核药物异烟肼的关键酶。上述两个发现使该化合物在辅助结核治疗的发展中具有潜在的优势。5-硝基噻吩部分首次被鉴定为分枝杆菌芳胺N-乙酰基转移酶的前瞻性抑制剂支架,这是代谢一线抗结核药物异烟肼的关键酶。上述两个发现使该化合物在辅助结核治疗的发展中具有潜在的优势。5-硝基噻吩部分首次被鉴定为分枝杆菌芳胺N-乙酰基转移酶的前瞻性抑制剂支架,这是代谢一线抗结核药物异烟肼的关键酶。上述两个发现使该化合物在辅助结核治疗的发展中具有潜在的优势。






























 京公网安备 11010802027423号
京公网安备 11010802027423号