当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photochemistry of 2-Nitroarenes: 2-Nitrophenyl-α-trifluoromethyl Carbinols as Synthons for Fluoroorganics
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-09-26 , DOI: 10.1021/jacs.9b07241 Kavita Belligund 1, 2 , Thomas Mathew 1, 2 , Jonathan R Hunt 2 , Archith Nirmalchandar 1, 2 , Ralf Haiges 1, 2 , Jahan Dawlaty 2 , G K Surya Prakash 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2019-09-26 , DOI: 10.1021/jacs.9b07241 Kavita Belligund 1, 2 , Thomas Mathew 1, 2 , Jonathan R Hunt 2 , Archith Nirmalchandar 1, 2 , Ralf Haiges 1, 2 , Jahan Dawlaty 2 , G K Surya Prakash 1, 2
Affiliation
|
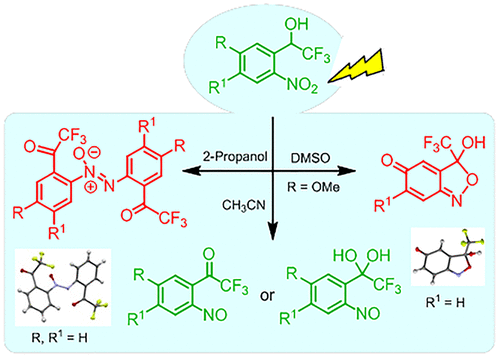
Facile synthesis of a new series of 2,2'-bis(trifluoroacetyl) azoxybenzene derivatives and trifluoromethylated benzo[c]isoxazoline systems, along with trifluoroacetyl nitrosobenzene derivatives was achieved by solvent controlled photolysis of appropriate 2-nitrobenzyl alcohols. Corresponding photoactive 2-nitrobenzyl chromophore plays a distinct role in this photosynthetic process, while, quite unprecedented, pertinent fluoromethyl substitution leads to high value fluoromethylated products, whose direct access is not feasible by common synthetic protocols. The significance of fluorine and fluoroalkyl substitution and its prominent biological effects makes this new photochemical approach an important discovery in synthetic methodology. Plausible mechanistic pathways involved in the formation of the products during steady-state photolysis are further established by picosecond laser flash photolysis experiments.
中文翻译:

2-硝基芳烃的光化学:2-硝基苯基-α-三氟甲基甲醇作为含氟有机物的合成子
通过适当的 2-硝基苯甲醇的溶剂控制光解,可以轻松合成一系列新的 2,2'-双(三氟乙酰基)偶氮苯衍生物和三氟甲基化苯并 [c] 异恶唑啉系统,以及三氟乙酰亚硝基苯衍生物。相应的光活性 2-硝基苄基发色团在该光合作用过程中发挥着独特的作用,而前所未有的相关氟甲基取代导致高价值的氟甲基化产品,其直接访问是通过常见的合成协议不可行的。氟和氟烷基取代的重要性及其突出的生物学效应使这种新的光化学方法成为合成方法学中的重要发现。
更新日期:2019-09-26
中文翻译:

2-硝基芳烃的光化学:2-硝基苯基-α-三氟甲基甲醇作为含氟有机物的合成子
通过适当的 2-硝基苯甲醇的溶剂控制光解,可以轻松合成一系列新的 2,2'-双(三氟乙酰基)偶氮苯衍生物和三氟甲基化苯并 [c] 异恶唑啉系统,以及三氟乙酰亚硝基苯衍生物。相应的光活性 2-硝基苄基发色团在该光合作用过程中发挥着独特的作用,而前所未有的相关氟甲基取代导致高价值的氟甲基化产品,其直接访问是通过常见的合成协议不可行的。氟和氟烷基取代的重要性及其突出的生物学效应使这种新的光化学方法成为合成方法学中的重要发现。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号