当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis and biological evaluation of new multi-target 3-(1H-indol-3-yl)pyrrolidine-2,5-dione derivatives with potential antidepressant effect.
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-09-26 , DOI: 10.1016/j.ejmech.2019.111736 Martyna Z Wróbel 1 , Andrzej Chodkowski 1 , Franciszek Herold 1 , Monika Marciniak 1 , Maciej Dawidowski 1 , Agata Siwek 2 , Gabriela Starowicz 2 , Katarzyna Stachowicz 3 , Bernadeta Szewczyk 3 , Gabriel Nowak 4 , Mariusz Belka 5 , Tomasz Bączek 5 , Grzegorz Satała 6 , Andrzej J Bojarski 6 , Jadwiga Turło 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2019-09-26 , DOI: 10.1016/j.ejmech.2019.111736 Martyna Z Wróbel 1 , Andrzej Chodkowski 1 , Franciszek Herold 1 , Monika Marciniak 1 , Maciej Dawidowski 1 , Agata Siwek 2 , Gabriela Starowicz 2 , Katarzyna Stachowicz 3 , Bernadeta Szewczyk 3 , Gabriel Nowak 4 , Mariusz Belka 5 , Tomasz Bączek 5 , Grzegorz Satała 6 , Andrzej J Bojarski 6 , Jadwiga Turło 1
Affiliation
|
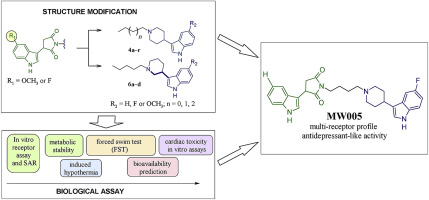
A series of novel 3-(1H-indol-3-yl)pyrrolidine-2,5-dione derivatives were synthesised and evaluated for their 5-HT1A/D2/5-HT2A/5-HT6/5-HT7 receptor affinity and serotonin reuptake inhibition. Most of the evaluated compounds displayed high affinities for 5-HT1A receptors (e.g., 4cKi = 2.3 nM, 4lKi = 3.2 nM). The antidepressant activity of the selected compounds was screened in vivo using the forced swim test (FST). The results indicate that compound MW005 (agonist of the pre- and postsynaptic 5-HT1A receptor) exhibited promising affinities for the 5-HT1A/SERT/D2/5-HT6/5-HT7 receptors and showed an antidepressant-like activity in the FST model.
中文翻译:

具有潜在抗抑郁作用的新型多目标3-(1H-吲哚-3-基)吡咯烷-2,5-二酮衍生物的合成和生物学评价。
合成了一系列新颖的3-(1H-吲哚-3-基)吡咯烷-2,5-二酮衍生物并评估了它们的5-HT1A / D2 / 5-HT2A / 5-HT6 / 5-HT7受体亲和力和血清素再摄取抑制。大多数评估的化合物显示出对5-HT1A受体的高亲和力(例如4cKi = 2.3 nM,4lKi = 3.2 nM)。使用强制游泳测试(FST)在体内筛选了所选化合物的抗抑郁活性。结果表明,化合物MW005(突触前5-HT1A受体激动剂)对5-HT1A / SERT / D2 / 5-HT6 / 5-HT7受体表现出有希望的亲和力,并在FST中表现出抗抑郁样活性模型。
更新日期:2019-09-26
中文翻译:

具有潜在抗抑郁作用的新型多目标3-(1H-吲哚-3-基)吡咯烷-2,5-二酮衍生物的合成和生物学评价。
合成了一系列新颖的3-(1H-吲哚-3-基)吡咯烷-2,5-二酮衍生物并评估了它们的5-HT1A / D2 / 5-HT2A / 5-HT6 / 5-HT7受体亲和力和血清素再摄取抑制。大多数评估的化合物显示出对5-HT1A受体的高亲和力(例如4cKi = 2.3 nM,4lKi = 3.2 nM)。使用强制游泳测试(FST)在体内筛选了所选化合物的抗抑郁活性。结果表明,化合物MW005(突触前5-HT1A受体激动剂)对5-HT1A / SERT / D2 / 5-HT6 / 5-HT7受体表现出有希望的亲和力,并在FST中表现出抗抑郁样活性模型。








































 京公网安备 11010802027423号
京公网安备 11010802027423号