Electrochimica Acta ( IF 5.5 ) Pub Date : 2019-09-25 , DOI: 10.1016/j.electacta.2019.134952 Rana Pratap Singh , Payal Arora , Subramanian Nellaiappan , C. Shivakumara , Silvia Irusta , Manas Paliwal , Sudhanshu Sharma
|
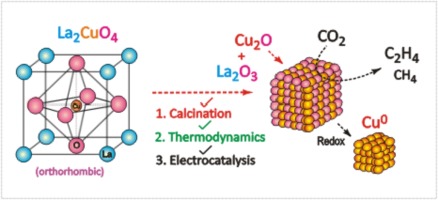
La2CuO4, a layered perovskites oxide was synthesized using solution combustion method at different calcination temperatures. XRD characterization and Rietveld refinement analysis confirms the pure phase of the synthesized material. The effect of calcination causes shrinking in the lattice parameters affirm the change in the oxygen stoichiometry. Four Probe (Van der Pauw’s) and CP-AFM analysis were carried out to understand the influences of calcination temperature on electrical conductivity. Electrochemical investigations on La2CuO4 were performed at various negative potential ranges using cyclic voltammetry (CV) to identify the redox active centre. Thermodynamic calculations are further carried out to validate the electrochemical results in different mediums. Selective electrocatalytic conversion of CO2 to C2-hydrocarbon (C2H4) with FE% of 40.3% which is 10 times higher than CH4 (FE% = 4.1%) emphasized the in-situ formation of Cu2O from the redox active centre present in La2CuO4. Hydrocarbons are generated at a very low overvoltage (−0.4 V RHE) in comparison to literature. XPS analysis indeed confirms the presence of Cu+ species i.e., in-situ generated Cu2O, on La2CuO4 after CV and CO2 electrocatalysis.
中文翻译:

层状La 2 CuO 4钙钛矿的电化学见解:活性离子铜,可在低过电势下选择性还原CO 2
采用固溶燃烧法在不同的煅烧温度下合成了La 2 CuO 4,为层状钙钛矿氧化物。XRD表征和Rietveld精炼分析证实了合成材料的纯相。煅烧的影响导致晶格参数的缩小,证实了氧化学计量的变化。进行了四探针(Van der Pauw's)和CP-AFM分析,以了解煅烧温度对电导率的影响。La 2 CuO 4的电化学研究使用循环伏安法(CV)在各种负电位范围内进行氧化还原,以鉴定氧化还原活性中心。进一步进行热力学计算以验证在不同介质中的电化学结果。具有20.3%的FE%的CO 2选择性电催化转化为C 2-碳氢化合物(C 2 H 4),是CH 4(FE%= 4.1%)的10倍,这强调了Cu 2 O的原位形成。La 2 CuO 4中存在氧化还原活性中心。与文献相比,碳氢化合物以非常低的过压(-0.4 V RHE)生成。XPS分析确实证实了铜的存在+物种,即,在原位生成的Cu 2 O,上拉2的CuO 4 CV和CO后2电催化。






























 京公网安备 11010802027423号
京公网安备 11010802027423号