当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elucidating Acidic Electro-Oxidation Pathways of Furfural on Platinum
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-10-16 , DOI: 10.1021/acscatal.9b02656 Alex M. Román , Joseph C. Hasse , J. Will Medlin , Adam Holewinski
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-10-16 , DOI: 10.1021/acscatal.9b02656 Alex M. Román , Joseph C. Hasse , J. Will Medlin , Adam Holewinski
|
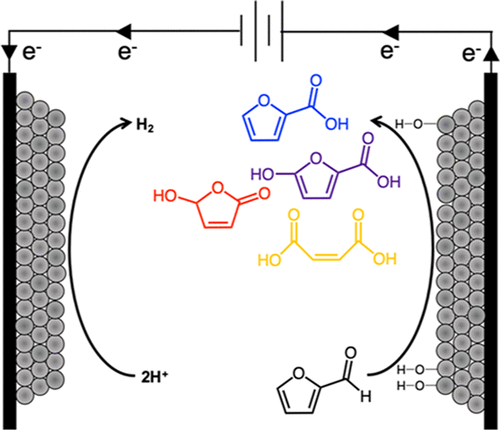
Electrocatalysis poses a number of possible advantages for the valorization of biomass-derived feedstocks, most critically its amenability to direct conversion in acidic aqueous media. Furanic biomass derivatives, such as furfural, are substrates with a number of value-added chemical outlets via partial oxidation, most notably furoic acid (FA), a potential precursor to 2,5-furandicarboxylic acid (FDCA). Pairing such partial oxidations with H2 evolution or other reduction reactions (e.g. CO2) in an electrochemical cell presents an opportunity to perform electrolysis at lowered voltages, while coproducing products that are more valuable than O2. Here, we have utilized differential reactor studies with online electrochemical mass spectrometry (OLEMS), as well as in situ infrared spectroscopy attenuated total reflectance-surface-enhanced infrared reflection-absorption spectroscopy (ATR-SEIRAS), to probe the oxidative reaction pathways of furfural on platinum catalysts in acidic electrolyte. We find furfural electro-oxidation selectivity to depend on potential, with the largest shifts corresponding to the transition of Pt to Pt-oxide. Below 1.2 VRHE, FA and 5-hydroxyfuroic acid are the primary products. At higher potential, selectivity shifts predominantly toward 5-hydroxy-furan-2(5H)-one (HFN), with the appearance of maleic acid (MA) as well. ATR-SEIRAS and OLEMS indicate that decomposition and overoxidation to CO2 occurs via decarbonylated or decarboxylated ring intermediates, while MA is less easily activated toward further oxidation once it is formed. Significant oxidative currents are only achieved at potentials where the surface is cleared of CO, which is derived from spontaneous decarbonylation of furfural on the metallic Pt surface. As potential is increased, selectivity to C4 and C5 oxygenates over CO2 is then promoted by a high steady-state surface coverage of organic intermediates that inhibit rapid adsorption and addition of oxygen from the discharge of water. Based on these findings, we propose a reaction pathway and directions for the design of more active and selective electrocatalysts.
中文翻译:

阐明铂上糠醛的酸性电氧化途径
电催化作用为生物质衍生的原料的增值提供了许多可能的优势,最关键的是它具有在酸性水性介质中直接转化的能力。呋喃生物质衍生物(例如糠醛)是通过部分氧化而具有大量增值化学出口的底物,最著名的是呋喃甲酸(FA),它是2,5-呋喃二甲酸(FDCA)的潜在前体。在电化学电池中将这种部分氧化与H 2析出或其他还原反应(例如CO 2)结合使用,可提供在较低电压下进行电解的机会,同时可生产比O 2更有价值的产品。在这里,我们利用在线电化学质谱(OLEMS)的差动反应器研究以及原位红外光谱衰减全反射率表面增强的红外反射吸收光谱(ATR-SEIRAS),来探索糠醛的氧化反应途径在酸性电解质中的铂催化剂上。我们发现糠醛电氧化选择性取决于电势,最大位移对应于Pt到Pt氧化物的跃迁。低于1.2 V RHE,FA和5-羟基糠酸是主要产物。在较高的电势下,选择性也主要向5-羟基-呋喃-2(5 H)-one(HFN)转移,并且也出现了顺丁烯二酸(MA)。ATR-SEIRAS和OLEMS表明分解和过氧化为CO2通过脱羰或脱羧环中间体发生,而MA一旦形成则不易被活化而进一步氧化。仅在表面清除了CO的电势下才能实现明显的氧化电流,CO源自金属Pt表面上糠醛的自发脱羰作用。随着电势的增加,有机中间体的高稳态表面覆盖率会提高C 4和C 5含氧化合物对CO 2的选择性,而有机中间体的稳态表面覆盖会抑制水的快速吸附和氧从排水中的添加。基于这些发现,我们提出了设计更具活性和选择性的电催化剂的反应途径和方向。
更新日期:2019-10-17
中文翻译:

阐明铂上糠醛的酸性电氧化途径
电催化作用为生物质衍生的原料的增值提供了许多可能的优势,最关键的是它具有在酸性水性介质中直接转化的能力。呋喃生物质衍生物(例如糠醛)是通过部分氧化而具有大量增值化学出口的底物,最著名的是呋喃甲酸(FA),它是2,5-呋喃二甲酸(FDCA)的潜在前体。在电化学电池中将这种部分氧化与H 2析出或其他还原反应(例如CO 2)结合使用,可提供在较低电压下进行电解的机会,同时可生产比O 2更有价值的产品。在这里,我们利用在线电化学质谱(OLEMS)的差动反应器研究以及原位红外光谱衰减全反射率表面增强的红外反射吸收光谱(ATR-SEIRAS),来探索糠醛的氧化反应途径在酸性电解质中的铂催化剂上。我们发现糠醛电氧化选择性取决于电势,最大位移对应于Pt到Pt氧化物的跃迁。低于1.2 V RHE,FA和5-羟基糠酸是主要产物。在较高的电势下,选择性也主要向5-羟基-呋喃-2(5 H)-one(HFN)转移,并且也出现了顺丁烯二酸(MA)。ATR-SEIRAS和OLEMS表明分解和过氧化为CO2通过脱羰或脱羧环中间体发生,而MA一旦形成则不易被活化而进一步氧化。仅在表面清除了CO的电势下才能实现明显的氧化电流,CO源自金属Pt表面上糠醛的自发脱羰作用。随着电势的增加,有机中间体的高稳态表面覆盖率会提高C 4和C 5含氧化合物对CO 2的选择性,而有机中间体的稳态表面覆盖会抑制水的快速吸附和氧从排水中的添加。基于这些发现,我们提出了设计更具活性和选择性的电催化剂的反应途径和方向。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号