当前位置:
X-MOL 学术
›
Asian J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1,10‐Phenanthroline Carboxylic Acids for Preparation of Functionalized Metal‐Organic Frameworks
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2019-10-16 , DOI: 10.1002/ajoc.201900569 Anton S. Abel 1, 2 , Alexander Yu Mitrofanov 1, 2 , Aleksei A. Yakushev 1 , Ilya S. Zenkov 1, 2 , Gleb V. Morozkov 1 , Alexei D. Averin 1, 3 , Irina P. Beletskaya 1, 3 , Julien Michalak 2 , Stéphane Brandès 2 , Alla Bessmertnykh‐Lemeune 2
Asian Journal of Organic Chemistry ( IF 2.8 ) Pub Date : 2019-10-16 , DOI: 10.1002/ajoc.201900569 Anton S. Abel 1, 2 , Alexander Yu Mitrofanov 1, 2 , Aleksei A. Yakushev 1 , Ilya S. Zenkov 1, 2 , Gleb V. Morozkov 1 , Alexei D. Averin 1, 3 , Irina P. Beletskaya 1, 3 , Julien Michalak 2 , Stéphane Brandès 2 , Alla Bessmertnykh‐Lemeune 2
Affiliation
|
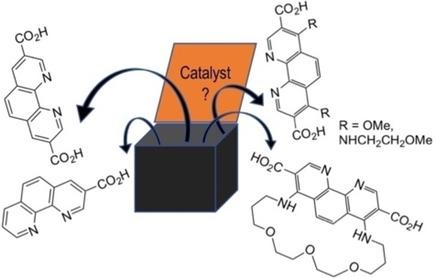
Synthetic approaches to 1,10‐phenanthroline‐3‐carboxylic acid (2), 1,10‐phenanthroline‐3,8‐dicarboxylic acid (3) and their functionalized derivatives were investigated. Acids 2 and 3 were prepared in good yields from bromophenanthrolines via palladium‐catalyzed alkoxycarbonylation. Moreover, butyl 8‐bromo‐1,10‐phenanthroline‐3‐carboxylate was obtained in acceptable yield (25–35%) by ceasing the carbonylation of the dibromide 5 after 30–70% consumption of the starting compound. To prepare functionalized derivatives of acids 2 and 3, the reactions of butyl 8‐bromo‐1,10‐phenanthroline‐3‐carboxylate and diethyl 4,7‐dichloro‐1,10‐phenanthroline‐3,8‐dicarboxylate with various nucleophiles were investigated. SNAr reactions are suitable for the synthesis of 4,7‐diazido‐, dimethoxy‐ and diamino‐substituted 3,8‐bis(ethoxycarbonyl)phenanthrolines, including the macrocyclic derivatives. The bromine atom at position 8 of the phenanthroline ring reacts with nucleophiles only in the presence of the palladium catalysts. The scope of these reactions was briefly investigated conducting Sonogashira, Suzuki‐Miyaura and Hirao reactions. Hydrolysis of the functionalized esters of phenanthroline leads to corresponding acids in good yields.
中文翻译:

1,10-菲咯啉羧酸用于制备功能化的金属有机骨架
研究了1,10-菲咯啉-3-羧酸(2),1,10-菲咯啉-3,8-二羧酸(3)及其官能化衍生物的合成方法。溴代菲咯啉经钯催化的烷氧羰基化反应可制得高产的酸2和3。此外,在消耗30-70%的起始化合物后,通过停止二溴化物5的羰基化反应,可以以可接受的收率(25-35%)获得8-溴-1,10-菲咯啉-3-羧酸丁酯。制备酸2和3的官能化衍生物,研究了8-溴-1,10-菲咯啉-3-羧酸丁酯和4,7-二氯-1,10-菲咯啉-3,8-二羧酸二乙酯与各种亲核试剂的反应。S N Ar反应适用于合成4,7-二叠氮基,二甲氧基和二氨基取代的3,8-双(乙氧羰基)菲咯啉,包括大环衍生物。菲咯啉环第8位的溴原子仅在钯催化剂存在下才与亲核试剂反应。对这些反应的范围进行了简要的研究,以进行Sonogashira,Suzuki-Miyaura和Hirao反应。菲咯啉的官能化酯的水解以良好的产率产生相应的酸。
更新日期:2019-10-17
中文翻译:

1,10-菲咯啉羧酸用于制备功能化的金属有机骨架
研究了1,10-菲咯啉-3-羧酸(2),1,10-菲咯啉-3,8-二羧酸(3)及其官能化衍生物的合成方法。溴代菲咯啉经钯催化的烷氧羰基化反应可制得高产的酸2和3。此外,在消耗30-70%的起始化合物后,通过停止二溴化物5的羰基化反应,可以以可接受的收率(25-35%)获得8-溴-1,10-菲咯啉-3-羧酸丁酯。制备酸2和3的官能化衍生物,研究了8-溴-1,10-菲咯啉-3-羧酸丁酯和4,7-二氯-1,10-菲咯啉-3,8-二羧酸二乙酯与各种亲核试剂的反应。S N Ar反应适用于合成4,7-二叠氮基,二甲氧基和二氨基取代的3,8-双(乙氧羰基)菲咯啉,包括大环衍生物。菲咯啉环第8位的溴原子仅在钯催化剂存在下才与亲核试剂反应。对这些反应的范围进行了简要的研究,以进行Sonogashira,Suzuki-Miyaura和Hirao反应。菲咯啉的官能化酯的水解以良好的产率产生相应的酸。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号