当前位置:
X-MOL 学术
›
J. Phys. Chem. C
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nanostructured Tungstate-Derived Copper for Hydrogen Evolution Reaction and Electroreduction of CO2 in Sodium Hydroxide Solutions
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-10-10 , DOI: 10.1021/acs.jpcc.9b07133
Hossein Farsi , Shokufeh Moghiminia , Majid Raygan , Elahe Dana , Seyyedamirhossein Hosseini , Mitra Behforooz , Tykhon Zubkov , Ian V. Lightcap 1 , Zhihai Li
The Journal of Physical Chemistry C ( IF 3.3 ) Pub Date : 2019-10-10 , DOI: 10.1021/acs.jpcc.9b07133
Hossein Farsi , Shokufeh Moghiminia , Majid Raygan , Elahe Dana , Seyyedamirhossein Hosseini , Mitra Behforooz , Tykhon Zubkov , Ian V. Lightcap 1 , Zhihai Li
Affiliation
|
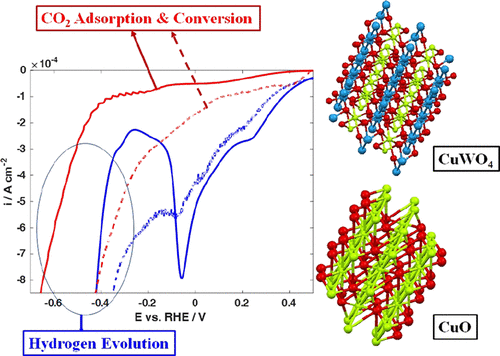
Electroreduction of CO2 became an important topic recently because it can reduce the atmospheric CO2 levels and simultaneously synthesize chemical fuels. However, efficient conversion of CO2 to produce fuels remains a challenge because a proper electrocatalyst is needed to make this CO2 reduction process more selective and efficient. In this study, we prepared nanostructured tungstate-derived copper to test its application in CO2 reduction. The prepared copper tungstate (CuWO4) nanomaterials were first characterized by analytical techniques such as transmission electron microscopy, X-ray diffraction, and X-ray photoelectron spectroscopy to determine the particle size, crystallinity, purity, and composition. Then, the CuWO4 nanomaterials were further investigated in an aqueous solution containing 0.1 M NaOH by electrochemical cyclic voltammetry (CV) and linear sweep voltammetry (LSV) techniques. The CO2 electroreduction experiments were carried out in 0.1 M NaOH with the presence of CO2, and the analysis of electrochemical results shows that nanostructured CuWO4 performs better in comparison with CuO—a well-known electrocatalyst for reducing CO2 to nongaseous carbon-containing products such as alcohols—because of poisoning effects of adsorbed CO2 or its adsorbed–reduced intermediates on hydrogen evolution reaction. Our results also show that CO2-reduction intermediates adsorbed strongly on the surface of CuWO4, which increases the overpotential for hydrogen evolution reaction on the surface of CuWO4 by as much as 230 mV against the 70 mV for CuO, at a current density of 0.8 mA cm–2.
中文翻译:

纳米结构钨酸铜在氢氧化钠溶液中的析氢反应和CO 2的电还原
CO的电还原2最近成为一个重要的话题,因为它可以减少大气中的二氧化碳2的水平,同时合成化学燃料。然而,由于需要合适的电催化剂来使该CO 2还原过程更具选择性和效率,因此有效地将CO 2转化为燃料仍然是一个挑战。在这项研究中,我们制备了纳米结构的钨酸铜,以测试其在CO 2还原中的应用。制备的钨酸铜(CuWO 4纳米材料首先通过分析技术进行表征,例如透射电子显微镜,X射线衍射和X射线光电子能谱,以确定粒径,结晶度,纯度和组成。然后,通过电化学循环伏安法(CV)和线性扫描伏安法(LSV)技术进一步研究了CuWO 4纳米材料在含有0.1 M NaOH的水溶液中的作用。该CO 2电还原实验在0.1M NaOH中进行与CO的存在2,和电化学结果示出了分析该纳米结构化的钨酸铜4点进行更好与CuO系的公知电比较用于减少CO 2到非气态含碳产物(如醇)中,因为吸附的CO 2或其吸附的还原性中间体对氢释放反应有毒害作用。我们的结果还表明,CO 2 -还原的中间体钨酸铜的表面上牢固地吸附4,这增加了超电势析氢反应钨酸铜的表面上4多达230毫伏针对70毫伏的CuO,在电流密度的0.8 mA cm –2。
更新日期:2019-10-10
中文翻译:

纳米结构钨酸铜在氢氧化钠溶液中的析氢反应和CO 2的电还原
CO的电还原2最近成为一个重要的话题,因为它可以减少大气中的二氧化碳2的水平,同时合成化学燃料。然而,由于需要合适的电催化剂来使该CO 2还原过程更具选择性和效率,因此有效地将CO 2转化为燃料仍然是一个挑战。在这项研究中,我们制备了纳米结构的钨酸铜,以测试其在CO 2还原中的应用。制备的钨酸铜(CuWO 4纳米材料首先通过分析技术进行表征,例如透射电子显微镜,X射线衍射和X射线光电子能谱,以确定粒径,结晶度,纯度和组成。然后,通过电化学循环伏安法(CV)和线性扫描伏安法(LSV)技术进一步研究了CuWO 4纳米材料在含有0.1 M NaOH的水溶液中的作用。该CO 2电还原实验在0.1M NaOH中进行与CO的存在2,和电化学结果示出了分析该纳米结构化的钨酸铜4点进行更好与CuO系的公知电比较用于减少CO 2到非气态含碳产物(如醇)中,因为吸附的CO 2或其吸附的还原性中间体对氢释放反应有毒害作用。我们的结果还表明,CO 2 -还原的中间体钨酸铜的表面上牢固地吸附4,这增加了超电势析氢反应钨酸铜的表面上4多达230毫伏针对70毫伏的CuO,在电流密度的0.8 mA cm –2。

































 京公网安备 11010802027423号
京公网安备 11010802027423号