Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Crystal structure of heliorhodopsin
Nature ( IF 50.5 ) Pub Date : 2019-09-25 , DOI: 10.1038/s41586-019-1604-6
Wataru Shihoya , Keiichi Inoue , Manish Singh , Masae Konno , Shoko Hososhima , Keitaro Yamashita , Kento Ikeda , Akimitsu Higuchi , Tamaki Izume , Sae Okazaki , Masanori Hashimoto , Ritsu Mizutori , Sahoko Tomida , Yumeka Yamauchi , Rei Abe-Yoshizumi , Kota Katayama , Satoshi P. Tsunoda , Mikihiro Shibata , Yuji Furutani , Alina Pushkarev , Oded Béjà , Takayuki Uchihashi , Hideki Kandori , Osamu Nureki
Nature ( IF 50.5 ) Pub Date : 2019-09-25 , DOI: 10.1038/s41586-019-1604-6
Wataru Shihoya , Keiichi Inoue , Manish Singh , Masae Konno , Shoko Hososhima , Keitaro Yamashita , Kento Ikeda , Akimitsu Higuchi , Tamaki Izume , Sae Okazaki , Masanori Hashimoto , Ritsu Mizutori , Sahoko Tomida , Yumeka Yamauchi , Rei Abe-Yoshizumi , Kota Katayama , Satoshi P. Tsunoda , Mikihiro Shibata , Yuji Furutani , Alina Pushkarev , Oded Béjà , Takayuki Uchihashi , Hideki Kandori , Osamu Nureki
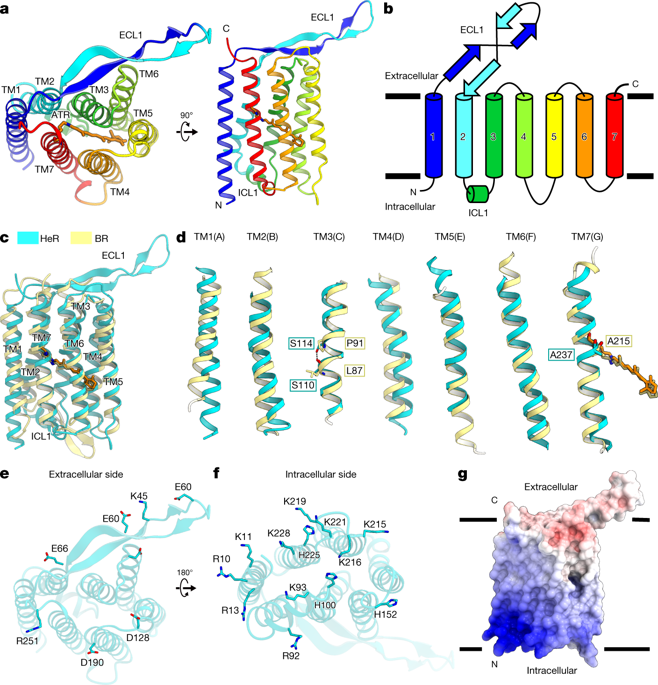
|
Heliorhodopsins (HeRs) are a family of rhodopsins that was recently discovered using functional metagenomics1. They are widely present in bacteria, archaea, algae and algal viruses2,3. Although HeRs have seven predicted transmembrane helices and an all-trans retinal chromophore as in the type-1 (microbial) rhodopsin, they display less than 15% sequence identity with type-1 and type-2 (animal) rhodopsins. HeRs also exhibit the reverse orientation in the membrane compared with the other rhodopsins. Owing to the lack of structural information, little is known about the overall fold and the photoactivation mechanism of HeRs. Here we present the 2.4-Å-resolution structure of HeR from an uncultured Thermoplasmatales archaeon SG8-52-1 (GenBank sequence ID LSSD01000000). Structural and biophysical analyses reveal the similarities and differences between HeRs and type-1 microbial rhodopsins. The overall fold of HeR is similar to that of bacteriorhodopsin. A linear hydrophobic pocket in HeR accommodates a retinal configuration and isomerization as in the type-1 rhodopsin, although most of the residues constituting the pocket are divergent. Hydrophobic residues fill the space in the extracellular half of HeR, preventing the permeation of protons and ions. The structure reveals an unexpected lateral fenestration above the β-ionone ring of the retinal chromophore, which has a critical role in capturing retinal from environment sources. Our study increases the understanding of the functions of HeRs, and the structural similarity and diversity among the microbial rhodopsins.A crystal structure of Thermoplasmatales archaeon heliorhodopsin at 2.4 Å resolution shows that it adopts a similar fold to that of type I rhodopsin—despite the low sequence identity—but there are also several marked differences that provide insights into heliorhodopsin function.
中文翻译:

日光视紫红质的晶体结构
Heliorhodopsins (HeRs) 是最近使用功能宏基因组学发现的一个视紫红质家族。它们广泛存在于细菌、古细菌、藻类和藻类病毒中2,3。尽管与 1 型(微生物)视紫质一样,HeR 具有 7 个预测的跨膜螺旋和全反式视网膜发色团,但它们与 1 型和 2 型(动物)视紫质的序列同一性不到 15%。与其他视紫红质相比,HeRs 在膜中也表现出相反的方向。由于缺乏结构信息,人们对 HeRs 的整体折叠和光活化机制知之甚少。在这里,我们展示了来自未培养的 Thermoplasmatales archaeon SG8-52-1(GenBank 序列 ID LSSD01000000)的 HeR 的 2.4 Å 分辨率结构。结构和生物物理分析揭示了 HeR 和 1 型微生物视紫红质之间的异同。HeR 的整体折叠与细菌视紫红质相似。HeR 中的线性疏水口袋容纳了 1 型视紫质中的视网膜构型和异构化,尽管构成口袋的大多数残基是不同的。疏水残基填充 HeR 细胞外一半的空间,防止质子和离子渗透。该结构揭示了视网膜发色团的 β-紫罗兰酮环上方出乎意料的横向开窗,这在从环境来源捕获视网膜方面具有关键作用。我们的研究增加了对 HeRs 功能的理解,以及微生物视紫红质之间的结构相似性和多样性。
更新日期:2019-09-25
中文翻译:

日光视紫红质的晶体结构
Heliorhodopsins (HeRs) 是最近使用功能宏基因组学发现的一个视紫红质家族。它们广泛存在于细菌、古细菌、藻类和藻类病毒中2,3。尽管与 1 型(微生物)视紫质一样,HeR 具有 7 个预测的跨膜螺旋和全反式视网膜发色团,但它们与 1 型和 2 型(动物)视紫质的序列同一性不到 15%。与其他视紫红质相比,HeRs 在膜中也表现出相反的方向。由于缺乏结构信息,人们对 HeRs 的整体折叠和光活化机制知之甚少。在这里,我们展示了来自未培养的 Thermoplasmatales archaeon SG8-52-1(GenBank 序列 ID LSSD01000000)的 HeR 的 2.4 Å 分辨率结构。结构和生物物理分析揭示了 HeR 和 1 型微生物视紫红质之间的异同。HeR 的整体折叠与细菌视紫红质相似。HeR 中的线性疏水口袋容纳了 1 型视紫质中的视网膜构型和异构化,尽管构成口袋的大多数残基是不同的。疏水残基填充 HeR 细胞外一半的空间,防止质子和离子渗透。该结构揭示了视网膜发色团的 β-紫罗兰酮环上方出乎意料的横向开窗,这在从环境来源捕获视网膜方面具有关键作用。我们的研究增加了对 HeRs 功能的理解,以及微生物视紫红质之间的结构相似性和多样性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号