当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Construction of Phenanthrenes and Chrysenes from β-Bromovinylarenes via Aryne Diels-Alder Reaction/Aromatization.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-10-14 , DOI: 10.1021/acs.joc.9b01644 Vikram Singh 1 , Ram Subhawan Verma 1 , Anil K Khatana 1 , Bhoopendra Tiwari 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-10-14 , DOI: 10.1021/acs.joc.9b01644 Vikram Singh 1 , Ram Subhawan Verma 1 , Anil K Khatana 1 , Bhoopendra Tiwari 1
Affiliation
|
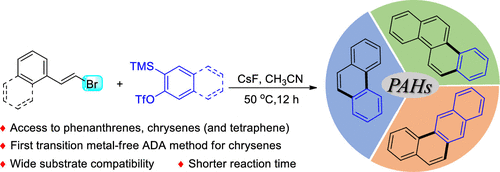
A highly efficient transition-metal-free general method for the synthesis of polycyclic aromatic hydrocarbons like phenanthrenes and chrysenes (and tetraphene) from β-bromovinylarenes and arynes has been developed. The reactions proceed via an aryne Diels-Alder (ADA) reaction, followed by a facile aromatization. This is the first report on direct construction of chrysenes (and tetraphene) using the ADA approach. Unlike the literature method which is limited to only 9/10-substituted derivatives, this method gives access to a wide variety of functionalized phenanthrenes.
中文翻译:

通过Aryne Diels-Alder反应/芳构化从β-溴乙烯基芳烃构建菲和萘。
已开发出一种高效的无过渡金属的通用方法,该方法可从β-溴乙烯基芳烃和芳烃中合成诸如菲和菲(及四苯)之类的多环芳烃。该反应通过芳烃Diels-Alder(ADA)反应进行,然后进行容易的芳构化。这是有关使用ADA方法直接构建车菊(和四苯酚)的第一份报告。与仅限于9/10取代衍生物的文献方法不同,该方法可使用多种官能化的菲。
更新日期:2019-10-14
中文翻译:

通过Aryne Diels-Alder反应/芳构化从β-溴乙烯基芳烃构建菲和萘。
已开发出一种高效的无过渡金属的通用方法,该方法可从β-溴乙烯基芳烃和芳烃中合成诸如菲和菲(及四苯)之类的多环芳烃。该反应通过芳烃Diels-Alder(ADA)反应进行,然后进行容易的芳构化。这是有关使用ADA方法直接构建车菊(和四苯酚)的第一份报告。与仅限于9/10取代衍生物的文献方法不同,该方法可使用多种官能化的菲。































 京公网安备 11010802027423号
京公网安备 11010802027423号