Cell Reports ( IF 7.5 ) Pub Date : 2019-09-24 , DOI: 10.1016/j.celrep.2019.08.066 Qiqi Li 1 , Qiuye Zhao 1 , Junyu Zhang 1 , Linkang Zhou 1 , Wenhao Zhang 1 , BoonTin Chua 2 , Yan Chen 3 , Li Xu 1 , Peng Li 1
|
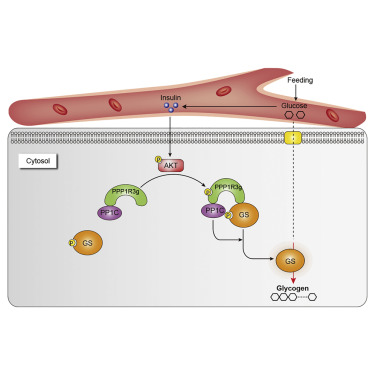
Insulin-stimulated hepatic glycogen synthesis is central to glucose homeostasis. Here, we show that PPP1R3G, a regulatory subunit of protein phosphatase 1 (PP1), is directly phosphorylated by AKT. PPP1R3G phosphorylation fluctuates with fasting-refeeding cycle and is required for insulin-stimulated dephosphorylation, i.e., activation of glycogen synthase (GS) in hepatocytes. In this study, we demonstrate that knockdown of PPP1R3G significantly inhibits insulin response. The introduction of wild-type PPP1R3G, and not phosphorylation-defective mutants, increases hepatic glycogen deposition, blood glucose clearance, and insulin sensitivity in vivo. Mechanistically, phosphorylated PPP1R3G displays increased binding for, and promotes dephosphorylation of, phospho-GS. Furthermore, PPP1R3B, another regulatory subunit of PP1, binds to the dephosphorylated GS, thereby relaying insulin stimulation to hepatic glycogen deposition. Importantly, this PP1-mediated signaling cascade is independent of GSK3. Therefore, we reveal a regulatory axis consisting of insulin/AKT/PPP1R3G/PPP1R3B that operates in parallel to the GSK3-dependent pathway, controlling glycogen synthesis and glucose homeostasis in insulin signaling.
中文翻译:

蛋白磷酸酶1复合物是AKT的直接靶标,它可将胰岛素信号转导至肝糖原沉积。
胰岛素刺激的肝糖原合成是葡萄糖稳态的关键。在这里,我们显示PPP1R3G,蛋白磷酸酶1(PP1)的调节亚基,被AKT直接磷酸化。PPP1R3G的磷酸化随禁食-再喂食周期而波动,是胰岛素刺激的去磷酸化(即肝细胞中糖原合酶(GS)的激活)所必需的。在这项研究中,我们证明敲低PPP1R3G可以显着抑制胰岛素反应。野生型PPP1R3G而非磷酸化缺陷型突变体的引入增加了体内肝糖原沉积,血糖清除率和胰岛素敏感性。从机理上讲,磷酸化的PPP1R3G与磷酸GS的结合力增强,并促进磷酸GS的去磷酸化。此外,PPP1R3B(PP1的另一个调节亚基)与去磷酸化的GS结合,从而将胰岛素刺激传递给肝糖原沉积。重要的是,此PP1介导的信号级联独立于GSK3。因此,我们揭示了一个由胰岛素/ AKT / PPP1R3G / PPP1R3B组成的调节轴,该轴与GSK3依赖性途径平行运作,控制胰岛素信号中的糖原合成和葡萄糖稳态。































 京公网安备 11010802027423号
京公网安备 11010802027423号