当前位置:
X-MOL 学术
›
Theranostics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Activatable Near-Infrared Chromophore for Multispectral Optoacoustic Imaging of Tumor Hypoxia and for Tumor Inhibition.
Theranostics ( IF 12.4 ) Pub Date : 2019-09-25 , DOI: 10.7150/thno.36755 Jing Huang 1 , Yinglong Wu 1 , Fang Zeng 1 , Shuizhu Wu 1
Theranostics ( IF 12.4 ) Pub Date : 2019-09-25 , DOI: 10.7150/thno.36755 Jing Huang 1 , Yinglong Wu 1 , Fang Zeng 1 , Shuizhu Wu 1
Affiliation
|
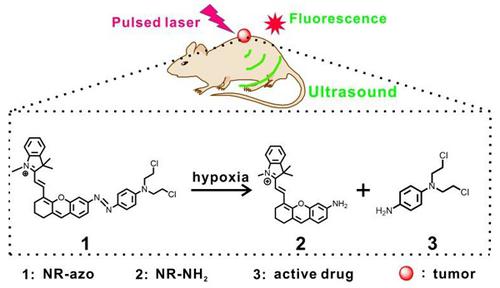
Hypoxia is a key hallmark of solid tumors and tumor hypoxia usually contributes to cancer progression, therapeutic resistance and poor outcome. Accurately detecting and imaging tumor hypoxia with high spatial resolution would be conducive to formulating optimized treatment plan and thus achieving better patient outcome. Methods: Tumor hypoxia can cleave the azo linker and release a NIR fluorophore (NR-NH2) and release the active drug as well. NR-NH2 shows a strong absorption band at around 680 nm and a strong fluorescence band at 710 nm, allowing for both multispectral optoacoustic tomography imaging (MSOT) and fluorescent imaging of tumor hypoxia in a tumor-bearing mouse model. Results: Liposome encapsulated with the activatable chromophore (NR-azo) for detecting/imaging tumor hypoxia and for tumor inhibition was demonstrated. For this chromophore, a xanthene-based NIR fluorophore acts as the optoacoustic and fluorescent reporter, an azo linker serves as the hypoxia-responsive moiety and a nitrogen mustard as the therapeutic drug. NR-azo shows an absorption at around 575 nm but exhibits negligible fluorescence due to the existence of the strong electron-withdrawing azo linker. Conclusion: We demonstrated an optoacoustic and fluorescent system for not only imaging tumor hypoxia in vivo but also achieving tumor inhibition.
中文翻译:

用于肿瘤缺氧多光谱光声成像和肿瘤抑制的可激活近红外发色团。
缺氧是实体瘤的一个关键标志,肿瘤缺氧通常会导致癌症进展、治疗耐药和不良预后。以高空间分辨率准确检测和成像肿瘤缺氧将有助于制定优化的治疗方案,从而实现更好的患者治疗效果。方法:肿瘤缺氧可以裂解偶氮接头并释放近红外荧光团(NR-NH2)并释放活性药物。NR-NH2 在 680 nm 附近显示出强吸收带,在 710 nm 处显示出强荧光带,可用于荷瘤小鼠模型中肿瘤缺氧的多光谱光声断层扫描成像 (MSOT) 和荧光成像。结果:证实了封装有可激活发色团(NR-偶氮)的脂质体可用于检测/成像肿瘤缺氧和抑制肿瘤。对于该发色团,基于呫吨的近红外荧光团充当光声和荧光报道分子,偶氮连接体充当缺氧响应部分,氮芥充当治疗药物。NR-azo 在 575 nm 附近显示出吸收,但由于存在强吸电子偶氮连接基,因此表现出可忽略不计的荧光。结论:我们展示了一种光声和荧光系统,不仅可以对体内肿瘤缺氧进行成像,而且可以实现肿瘤抑制。
更新日期:2019-09-25
中文翻译:

用于肿瘤缺氧多光谱光声成像和肿瘤抑制的可激活近红外发色团。
缺氧是实体瘤的一个关键标志,肿瘤缺氧通常会导致癌症进展、治疗耐药和不良预后。以高空间分辨率准确检测和成像肿瘤缺氧将有助于制定优化的治疗方案,从而实现更好的患者治疗效果。方法:肿瘤缺氧可以裂解偶氮接头并释放近红外荧光团(NR-NH2)并释放活性药物。NR-NH2 在 680 nm 附近显示出强吸收带,在 710 nm 处显示出强荧光带,可用于荷瘤小鼠模型中肿瘤缺氧的多光谱光声断层扫描成像 (MSOT) 和荧光成像。结果:证实了封装有可激活发色团(NR-偶氮)的脂质体可用于检测/成像肿瘤缺氧和抑制肿瘤。对于该发色团,基于呫吨的近红外荧光团充当光声和荧光报道分子,偶氮连接体充当缺氧响应部分,氮芥充当治疗药物。NR-azo 在 575 nm 附近显示出吸收,但由于存在强吸电子偶氮连接基,因此表现出可忽略不计的荧光。结论:我们展示了一种光声和荧光系统,不仅可以对体内肿瘤缺氧进行成像,而且可以实现肿瘤抑制。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号