当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Geometric E→Z Isomerisation of Alkenyl Silanes by Selective Energy Transfer Catalysis: Stereodivergent Synthesis of Triarylethylenes via a Formal anti-Metallometallation.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-10-31 , DOI: 10.1002/anie.201910169 Svenja I Faßbender 1 , John J Molloy 1 , Christian Mück-Lichtenfeld 1 , Ryan Gilmour 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-10-31 , DOI: 10.1002/anie.201910169 Svenja I Faßbender 1 , John J Molloy 1 , Christian Mück-Lichtenfeld 1 , Ryan Gilmour 1
Affiliation
|
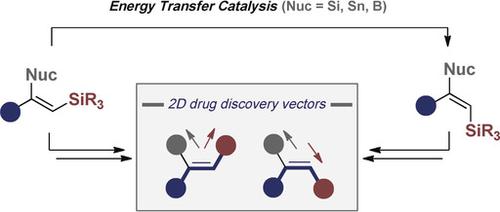
An efficient geometrical E→Z isomerisation of alkenyl silanes is disclosed via selective energy transfer using an inexpensive organic sensitiser. Characterised by operational simplicity, short reaction times (2 h), and broad substrate tolerance, the reaction displays high selectivity for trisubstituted systems (Z/E up to 95:5). In contrast to thermal activation, directionality results from deconjugation of the π-system in the Z-isomer due to A1,3 -strain thereby inhibiting re-activation. The structural importance of the β-substituent logically prompted an investigation of mixed bis-nucleophiles (Si, Sn, B). These versatile linchpins also undergo facile isomerisation, thereby enabling a formal anti-metallometallation. Mechanistic interrogation, supported by a theoretical investigation, is disclosed together with application of the products to the stereospecific synthesis of biologically relevant target structures.
中文翻译:

选择性能量转移催化烯基硅烷的几何 E→Z 异构化:通过形式反金属金属化立体发散合成三芳基乙烯。
公开了通过使用廉价有机敏化剂选择性能量转移实现烯基硅烷的有效几何E→Z异构化。该反应具有操作简单、反应时间短(2 小时)和广泛的底物耐受性等特点,对三取代体系表现出高选择性(Z/E 高达 95:5)。与热激活相反,方向性是由于 A1,3 应变导致 Z 异构体中 π 系统解共轭而产生的,从而抑制了重新激活。 β-取代基的结构重要性从逻辑上促进了对混合双亲核试剂(Si、Sn、B)的研究。这些多功能关键还可以进行轻松的异构化,从而实现正式的反金属金属化。公开了由理论研究支持的机械询问以及产品在生物相关目标结构的立体定向合成中的应用。
更新日期:2019-11-04
中文翻译:

选择性能量转移催化烯基硅烷的几何 E→Z 异构化:通过形式反金属金属化立体发散合成三芳基乙烯。
公开了通过使用廉价有机敏化剂选择性能量转移实现烯基硅烷的有效几何E→Z异构化。该反应具有操作简单、反应时间短(2 小时)和广泛的底物耐受性等特点,对三取代体系表现出高选择性(Z/E 高达 95:5)。与热激活相反,方向性是由于 A1,3 应变导致 Z 异构体中 π 系统解共轭而产生的,从而抑制了重新激活。 β-取代基的结构重要性从逻辑上促进了对混合双亲核试剂(Si、Sn、B)的研究。这些多功能关键还可以进行轻松的异构化,从而实现正式的反金属金属化。公开了由理论研究支持的机械询问以及产品在生物相关目标结构的立体定向合成中的应用。













































 京公网安备 11010802027423号
京公网安备 11010802027423号