当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Short Synthesis of the 2-Bromo-N,9-dimethyl-6,7,8,9-tetrahydro-5H-pyrido[2,3-b]indol-6-amine Building Block
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-09-27 , DOI: 10.1021/acs.oprd.9b00222 Nampally Sreenivasachary 1 , Heiko Kroth 1 , Pascal Benderitter 1 , Wolfgang Barth 1 , Andrea Pfeifer 1 , Andreas Muhs 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2019-09-27 , DOI: 10.1021/acs.oprd.9b00222 Nampally Sreenivasachary 1 , Heiko Kroth 1 , Pascal Benderitter 1 , Wolfgang Barth 1 , Andrea Pfeifer 1 , Andreas Muhs 1
Affiliation
|
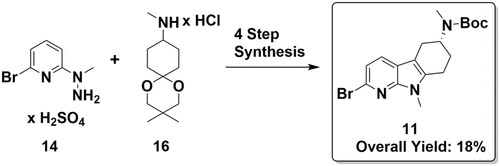
A concise synthesis of pharmaceutically useful (R)-tert-butyl N-(2-bromo-9-methyl-6,7,8,9-tetrahydro-5H-pyrido[2,3-b]indol-6-yl)-N-methylcarbamate building block 11 is described. The racemic intermediate 17 was prepared in a single step from 2-bromo-6-(1-methylhydrazinyl)pyridine sulfate salt (14) and N,3,3-trimethyl-1,5-dioxaspiro[5.5]undecan-9-amine hydrochloride salt (16). Chiral separation of racemic intermediate 17 by diasteromeric salt recrystallization afforded the diasteromeric salt 18 in 37% yield, which was Boc-protected to afford building block 11. Thus, the process for the synthesis and chiral separation by diasteromeric salt crystallization allowed the synthesis of chiral building block 11 in kilogram quantities in 18% overall yield.
中文翻译:

2-溴-N,9-二甲基-6,7,8,9-四氢-5 H-吡啶并[2,3 - b ]吲哚-6-胺结构单元的简短合成
药学上有用的(R)-叔丁基N-(2-溴-9-甲基-6,7,8,9-四氢-5 H-吡啶并[2,3 - b ]吲哚-6-基的简明合成)-描述了N-甲基氨基甲酸酯结构单元11。由2-溴-6-(1-甲基肼基)吡啶硫酸盐(14)和N,3,3-三甲基-1,5-二氧杂螺[5.5] undecan-9-胺一步一步制备外消旋中间体17盐酸盐(16)。通过非对映体盐重结晶手性分离外消旋中间体17,得到非对映体盐18收率为37%,这是受Boc保护的结构单元11。因此,通过非对映异构体盐结晶的合成和手性分离的方法允许以千克量合成手性结构单元11,总产率为18%。
更新日期:2019-09-27
中文翻译:

2-溴-N,9-二甲基-6,7,8,9-四氢-5 H-吡啶并[2,3 - b ]吲哚-6-胺结构单元的简短合成
药学上有用的(R)-叔丁基N-(2-溴-9-甲基-6,7,8,9-四氢-5 H-吡啶并[2,3 - b ]吲哚-6-基的简明合成)-描述了N-甲基氨基甲酸酯结构单元11。由2-溴-6-(1-甲基肼基)吡啶硫酸盐(14)和N,3,3-三甲基-1,5-二氧杂螺[5.5] undecan-9-胺一步一步制备外消旋中间体17盐酸盐(16)。通过非对映体盐重结晶手性分离外消旋中间体17,得到非对映体盐18收率为37%,这是受Boc保护的结构单元11。因此,通过非对映异构体盐结晶的合成和手性分离的方法允许以千克量合成手性结构单元11,总产率为18%。































 京公网安备 11010802027423号
京公网安备 11010802027423号