Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Preparation and Comparative Stability of a Kaolinite-Tetrabutylphosphonium Bromide Intercalation Compound for Heat and Solvent Treatments.
Langmuir ( IF 3.7 ) Pub Date : 2019-10-04 , DOI: 10.1021/acs.langmuir.9b02375 Shingo Machida , Régis Guégan , Yoshiyuki Sugahara 1
Langmuir ( IF 3.7 ) Pub Date : 2019-10-04 , DOI: 10.1021/acs.langmuir.9b02375 Shingo Machida , Régis Guégan , Yoshiyuki Sugahara 1
Affiliation
|
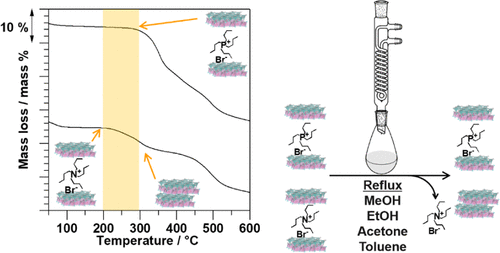
A kaolinite-tetrabutylphosphonium bromide (TBPBr) intercalation compound (Kaol-TBPBr) was prepared from kaolinite providing inorganic aluminosilicate layers and TBPBr as intercalated salts between the layers through the use of an intermediate, a kaolinite-dimethylsulfoxide (DMSO) intercalation compound (Kaol-DMSO). The experimental data through complementary techniques, including X-ray diffraction, Fourier transform infrared spectroscopy, solid-state 13C and 29Si nuclear magnetic resonance (NMR) spectroscopy with cross polarization and magic angle spinning, inductively coupled plasma emission spectrometry, and ion chromatography, indicate complete removal of DMSO and intercalation of TBPBr with an increase in the basal spacing from 1.12 nm (Kaol-DMSO) to 1.53 nm (Kaol-TBPBr). In contrast to a similar intercalation compound, a kaolinite-tetrabutylammonium bromide (TBABr) intercalation compound (Kaol-TBABr) with a basal spacing of 1.51 nm, Kaol-TBPBr displayed interesting features such as enhanced thermal stabilities as well as bold resistance against several solvents. Kaol-TBPBr withstood thermal decomposition of the organic species over 100 °C much better than Kaol-TBABr. When Kaol-TBPBr and Kaol-TBABr were refluxed in methanol, ethanol, acetone, or toluene for 1 day, Kaol-TBPBr preserved the expanded kaolinite layers, while the Kaol-TBABr structure completely collapsed due to the release of TBABr. Thus, with these particular and unique features of Kaol-TBPBr, organophosphonium salts appear to be promising guest species for intercalation chemistry of kaolinite.
中文翻译:

用于热处理和溶剂处理的高岭石-四丁基溴化phosph插层化合物的制备和比较稳定性。
高岭石-四丁基溴化phosph(TBPBr)插层化合物(Kaol-TBPBr)是通过使用中间体高岭石-二甲基亚砜(DMSO)插层化合物(Kaol- DMSO)。通过补充技术获得的实验数据表明,这些技术包括X射线衍射,傅立叶变换红外光谱,具有交叉极化和魔角旋转的固态13C和29Si核磁共振(NMR)光谱,电感耦合等离子体发射光谱和离子色谱,完全去除DMSO并插入TBPBr,并将基距从1.12 nm(Kaol-DMSO)增加到1.53 nm(Kaol-TBPBr)。与类似的插层化合物相比,一种基本间距为1.51 nm的高岭石-四丁基溴化铵(TBABr)插层化合物(Kaol-TBABr),Kaol-TBPBr具有有趣的功能,如增强的热稳定性以及对多种溶剂的大胆抵抗力。Kaol-TBPBr在100°C的温度下能抵抗有机物质的热分解,其性能比Kaol-TBABr好得多。当将Kaol-TBPBr和Kaol-TBABr在甲醇,乙醇,丙酮或甲苯中回流1天时,Kaol-TBPBr保留了膨胀的高岭石层,而由于TBABr的释放,Kaol-TBABr结构完全塌陷。因此,具有高岭土-TBPBr的这些特殊和独特的特征,有机phosph盐似乎是高岭石插层化学的有前途的客体。Kaol-TBPBr显示出有趣的功能,例如增强的热稳定性以及对多种溶剂的大胆抵抗力。Kaol-TBPBr在100°C的温度下能抵抗有机物质的热分解,其性能比Kaol-TBABr好得多。当将Kaol-TBPBr和Kaol-TBABr在甲醇,乙醇,丙酮或甲苯中回流1天时,Kaol-TBPBr保留了膨胀的高岭石层,而由于TBABr的释放,Kaol-TBABr结构完全塌陷。因此,具有高岭土-TBPBr的这些特殊和独特的特征,有机phosph盐似乎是高岭石插层化学的有前途的客体。Kaol-TBPBr显示出有趣的功能,例如增强的热稳定性以及对多种溶剂的大胆抵抗力。Kaol-TBPBr在100°C的温度下能抵抗有机物质的热分解,其性能比Kaol-TBABr好得多。当将Kaol-TBPBr和Kaol-TBABr在甲醇,乙醇,丙酮或甲苯中回流1天时,Kaol-TBPBr保留了膨胀的高岭石层,而由于TBABr的释放,Kaol-TBABr结构完全塌陷。因此,具有高岭土-TBPBr的这些特殊和独特的特征,有机phosph盐似乎是高岭石插层化学的有前途的客体。当将Kaol-TBPBr和Kaol-TBABr在甲醇,乙醇,丙酮或甲苯中回流1天时,Kaol-TBPBr保留了膨胀的高岭石层,而由于TBABr的释放,Kaol-TBABr结构完全塌陷。因此,具有高岭土-TBPBr的这些特殊和独特的特征,有机phosph盐似乎是高岭石插层化学的有前途的客体。当将Kaol-TBPBr和Kaol-TBABr在甲醇,乙醇,丙酮或甲苯中回流1天时,Kaol-TBPBr保留了膨胀的高岭石层,而由于TBABr的释放,Kaol-TBABr结构完全塌陷。因此,具有高岭土-TBPBr的这些特殊和独特的特征,有机phosph盐似乎是高岭石插层化学的有前途的客体。
更新日期:2019-10-04
中文翻译:

用于热处理和溶剂处理的高岭石-四丁基溴化phosph插层化合物的制备和比较稳定性。
高岭石-四丁基溴化phosph(TBPBr)插层化合物(Kaol-TBPBr)是通过使用中间体高岭石-二甲基亚砜(DMSO)插层化合物(Kaol- DMSO)。通过补充技术获得的实验数据表明,这些技术包括X射线衍射,傅立叶变换红外光谱,具有交叉极化和魔角旋转的固态13C和29Si核磁共振(NMR)光谱,电感耦合等离子体发射光谱和离子色谱,完全去除DMSO并插入TBPBr,并将基距从1.12 nm(Kaol-DMSO)增加到1.53 nm(Kaol-TBPBr)。与类似的插层化合物相比,一种基本间距为1.51 nm的高岭石-四丁基溴化铵(TBABr)插层化合物(Kaol-TBABr),Kaol-TBPBr具有有趣的功能,如增强的热稳定性以及对多种溶剂的大胆抵抗力。Kaol-TBPBr在100°C的温度下能抵抗有机物质的热分解,其性能比Kaol-TBABr好得多。当将Kaol-TBPBr和Kaol-TBABr在甲醇,乙醇,丙酮或甲苯中回流1天时,Kaol-TBPBr保留了膨胀的高岭石层,而由于TBABr的释放,Kaol-TBABr结构完全塌陷。因此,具有高岭土-TBPBr的这些特殊和独特的特征,有机phosph盐似乎是高岭石插层化学的有前途的客体。Kaol-TBPBr显示出有趣的功能,例如增强的热稳定性以及对多种溶剂的大胆抵抗力。Kaol-TBPBr在100°C的温度下能抵抗有机物质的热分解,其性能比Kaol-TBABr好得多。当将Kaol-TBPBr和Kaol-TBABr在甲醇,乙醇,丙酮或甲苯中回流1天时,Kaol-TBPBr保留了膨胀的高岭石层,而由于TBABr的释放,Kaol-TBABr结构完全塌陷。因此,具有高岭土-TBPBr的这些特殊和独特的特征,有机phosph盐似乎是高岭石插层化学的有前途的客体。Kaol-TBPBr显示出有趣的功能,例如增强的热稳定性以及对多种溶剂的大胆抵抗力。Kaol-TBPBr在100°C的温度下能抵抗有机物质的热分解,其性能比Kaol-TBABr好得多。当将Kaol-TBPBr和Kaol-TBABr在甲醇,乙醇,丙酮或甲苯中回流1天时,Kaol-TBPBr保留了膨胀的高岭石层,而由于TBABr的释放,Kaol-TBABr结构完全塌陷。因此,具有高岭土-TBPBr的这些特殊和独特的特征,有机phosph盐似乎是高岭石插层化学的有前途的客体。当将Kaol-TBPBr和Kaol-TBABr在甲醇,乙醇,丙酮或甲苯中回流1天时,Kaol-TBPBr保留了膨胀的高岭石层,而由于TBABr的释放,Kaol-TBABr结构完全塌陷。因此,具有高岭土-TBPBr的这些特殊和独特的特征,有机phosph盐似乎是高岭石插层化学的有前途的客体。当将Kaol-TBPBr和Kaol-TBABr在甲醇,乙醇,丙酮或甲苯中回流1天时,Kaol-TBPBr保留了膨胀的高岭石层,而由于TBABr的释放,Kaol-TBABr结构完全塌陷。因此,具有高岭土-TBPBr的这些特殊和独特的特征,有机phosph盐似乎是高岭石插层化学的有前途的客体。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号