当前位置:
X-MOL 学术
›
Nat. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regulation of the endosomal SNX27-retromer by OTULIN.
Nature Communications ( IF 14.7 ) Pub Date : 2019-09-20 , DOI: 10.1038/s41467-019-12309-z Aurelia Stangl 1 , Paul R Elliott 2, 3 , Adan Pinto-Fernandez 4 , Sarah Bonham 4 , Luke Harrison 5 , Annalisa Schaub 1 , Kerstin Kutzner 1 , Kirstin Keusekotten 2 , Paul T Pfluger 5, 6 , Farid El Oualid 7 , Benedikt M Kessler 4 , David Komander 2, 8 , Daniel Krappmann 1
Nature Communications ( IF 14.7 ) Pub Date : 2019-09-20 , DOI: 10.1038/s41467-019-12309-z Aurelia Stangl 1 , Paul R Elliott 2, 3 , Adan Pinto-Fernandez 4 , Sarah Bonham 4 , Luke Harrison 5 , Annalisa Schaub 1 , Kerstin Kutzner 1 , Kirstin Keusekotten 2 , Paul T Pfluger 5, 6 , Farid El Oualid 7 , Benedikt M Kessler 4 , David Komander 2, 8 , Daniel Krappmann 1
Affiliation
|
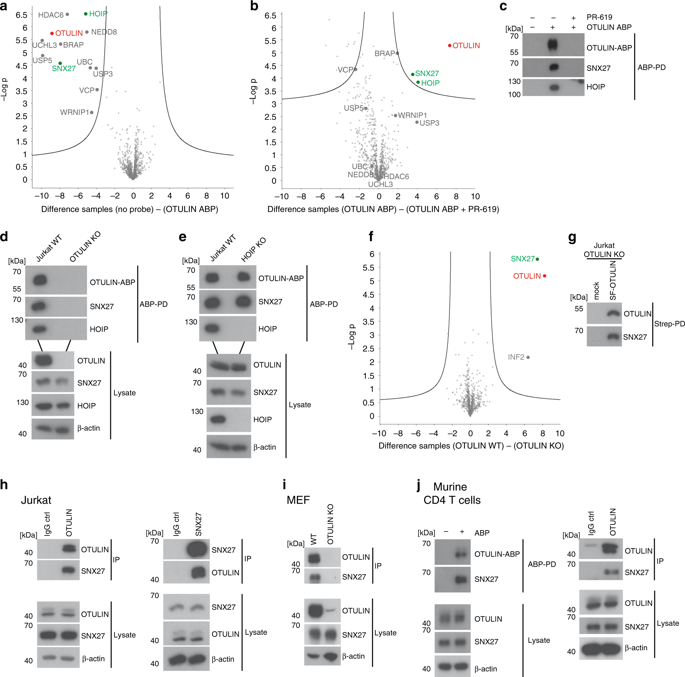
OTULIN (OTU Deubiquitinase With Linear Linkage Specificity) specifically hydrolyzes methionine1 (Met1)-linked ubiquitin chains conjugated by LUBAC (linear ubiquitin chain assembly complex). Here we report on the mass spectrometric identification of the OTULIN interactor SNX27 (sorting nexin 27), an adaptor of the endosomal retromer complex responsible for protein recycling to the cell surface. The C-terminal PDZ-binding motif (PDZbm) in OTULIN associates with the cargo-binding site in the PDZ domain of SNX27. By solving the structure of the OTU domain in complex with the PDZ domain, we demonstrate that a second interface contributes to the selective, high affinity interaction of OTULIN and SNX27. SNX27 does not affect OTULIN catalytic activity, OTULIN-LUBAC binding or Met1-linked ubiquitin chain homeostasis. However, via association, OTULIN antagonizes SNX27-dependent cargo loading, binding of SNX27 to the VPS26A-retromer subunit and endosome-to-plasma membrane trafficking. Thus, we define an additional, non-catalytic function of OTULIN in the regulation of SNX27-retromer assembly and recycling to the cell surface.
中文翻译:

OTULIN对内体SNX27-retromer的调节。
OTULIN(具有线性连接特异性的OTU去泛素酶)可特异性水解由LUBAC(线性泛素链装配复合体)缀合的蛋氨酸1(Met1)连接的泛素链。在这里,我们报告有关OTULIN相互作用物SNX27(分选nexin 27)的质谱鉴定,该物质是负责蛋白质循环到细胞表面的内体逆转录酶复合物的衔接子。OTULIN中的C端PDZ结合基序(PDZbm)与SNX27的PDZ域中的货物结合位点相关。通过解决与PDZ域复杂的OTU域的结构,我们证明了第二个接口有助于OTULIN和SNX27的选择性,高亲和力相互作用。SNX27不影响OTULIN催化活性,OTULIN-LUBAC结合或Met1连接的泛素链稳态。但是,通过关联,OTULIN拮抗依赖SNX27的货物装载,SNX27与VPS26A-retromer亚基的结合以及内体到质膜的运输。因此,我们定义了OTULIN在调节SNX27-retromer组装和回收到细胞表面中的其他非催化功能。
更新日期:2019-09-21
中文翻译:

OTULIN对内体SNX27-retromer的调节。
OTULIN(具有线性连接特异性的OTU去泛素酶)可特异性水解由LUBAC(线性泛素链装配复合体)缀合的蛋氨酸1(Met1)连接的泛素链。在这里,我们报告有关OTULIN相互作用物SNX27(分选nexin 27)的质谱鉴定,该物质是负责蛋白质循环到细胞表面的内体逆转录酶复合物的衔接子。OTULIN中的C端PDZ结合基序(PDZbm)与SNX27的PDZ域中的货物结合位点相关。通过解决与PDZ域复杂的OTU域的结构,我们证明了第二个接口有助于OTULIN和SNX27的选择性,高亲和力相互作用。SNX27不影响OTULIN催化活性,OTULIN-LUBAC结合或Met1连接的泛素链稳态。但是,通过关联,OTULIN拮抗依赖SNX27的货物装载,SNX27与VPS26A-retromer亚基的结合以及内体到质膜的运输。因此,我们定义了OTULIN在调节SNX27-retromer组装和回收到细胞表面中的其他非催化功能。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号