当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Theoretical Study of the Oxidation of Methane to Methanol by the [CuIICuII(μ-O)2CuIII(7-N-Etppz)]1+ Complex
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00054
Yan Fang Liu 1 , Likai Du 2
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2018-03-05 00:00:00 , DOI: 10.1021/acs.inorgchem.8b00054
Yan Fang Liu 1 , Likai Du 2
Affiliation
|
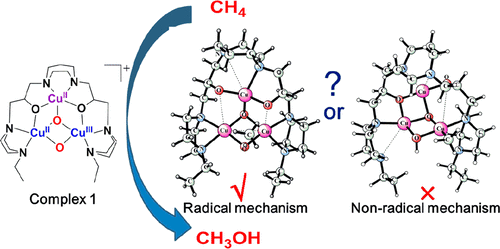
The reactivity patterns of a series of trivalent copper complexes have been studied to gain a better understanding of the chemical reactions occurring at the active site of particulate methane monooxygenase (pMMO). In this study, hybrid density functional theory is used to study the oxidation of methane to methanol mediated by the [CuIICuII(μ-O)2CuIII(7-N-Etppz)]1+ complex. Reaction mechanisms in different spin states were explored. Based on the calculated free-energy profile, a mechanism is suggested for the reaction of the oxidation of methane to methanol. The first step (1 → 2) is a hydrogen transfer to the bridged oxygen in the Cu2O2 core from the methane to form a methyl radical. The second step (2 → 3) is the radical recombination, in which the bridged hydroxyl rotates upward and exposes the oxygen moiety toward the methyl radical to form methanol. The radical recombination step is rate-limiting, with a calculated free-energy barrier of 19.6 kcal mol–1, which is in good agreement with the experimental value of 18.4 kcal mol–1. The mixed valent bis(μ-oxo)CuIICuIII species in the Cu3O4 core is directly responsible for the C–H activation of methane.
中文翻译:

[Cu II Cu II(μ-O)2 Cu III(7- N -Etppz)] 1+络合物将甲烷氧化为甲醇的理论研究
已经研究了一系列三价铜配合物的反应模式,以更好地了解在颗粒甲烷单加氧酶(pMMO)的活性位点发生的化学反应。在这项研究中,使用混合密度泛函理论研究由[Cu II Cu II(μ-O)2 Cu III(7- N -Etppz)] 1+络合物介导的甲烷氧化为甲醇的过程。探索了不同自旋态的反应机理。基于计算出的自由能分布,提出了甲烷氧化为甲醇的反应机理。第一步(1 → 2)是氢从甲烷转移到Cu 2 O 2核中的桥连氧上形成甲基。第二步(2 → 3)是自由基复合,其中桥连的羟基向上旋转并使氧部分朝向甲基自由基暴露,从而形成甲醇。自由基重组步骤是限速的,计算出的自由能垒为19.6 kcal mol –1,与实验值18.4 kcal mol –1很好地吻合。Cu 3 O 4核中的混合价双(μ-氧代)Cu II Cu III物种直接负责甲烷的CH–H活化。
更新日期:2018-03-05
中文翻译:

[Cu II Cu II(μ-O)2 Cu III(7- N -Etppz)] 1+络合物将甲烷氧化为甲醇的理论研究
已经研究了一系列三价铜配合物的反应模式,以更好地了解在颗粒甲烷单加氧酶(pMMO)的活性位点发生的化学反应。在这项研究中,使用混合密度泛函理论研究由[Cu II Cu II(μ-O)2 Cu III(7- N -Etppz)] 1+络合物介导的甲烷氧化为甲醇的过程。探索了不同自旋态的反应机理。基于计算出的自由能分布,提出了甲烷氧化为甲醇的反应机理。第一步(1 → 2)是氢从甲烷转移到Cu 2 O 2核中的桥连氧上形成甲基。第二步(2 → 3)是自由基复合,其中桥连的羟基向上旋转并使氧部分朝向甲基自由基暴露,从而形成甲醇。自由基重组步骤是限速的,计算出的自由能垒为19.6 kcal mol –1,与实验值18.4 kcal mol –1很好地吻合。Cu 3 O 4核中的混合价双(μ-氧代)Cu II Cu III物种直接负责甲烷的CH–H活化。

































 京公网安备 11010802027423号
京公网安备 11010802027423号