Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thiol-Mediated Multidentate Phosphorylcholine as a Zwitterionic Ligand for Stabilizing Biocompatible Gold Nanoparticles.
Langmuir ( IF 3.7 ) Pub Date : 2019-09-26 , DOI: 10.1021/acs.langmuir.9b01547 Wenya Zhou 1 , Longbing Ling 1 , Yawei Du 1 , Wei He 1 , Qing Xia 1 , Chen Yao 1 , Xinsong Li 1
Langmuir ( IF 3.7 ) Pub Date : 2019-09-26 , DOI: 10.1021/acs.langmuir.9b01547 Wenya Zhou 1 , Longbing Ling 1 , Yawei Du 1 , Wei He 1 , Qing Xia 1 , Chen Yao 1 , Xinsong Li 1
Affiliation
|
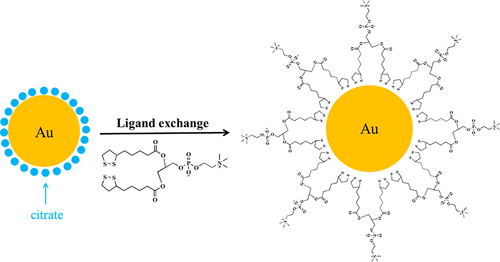
The increasing application of gold nanoparticles (AuNPs) in biomedicine requires extensive investigation of surface modification and stabilization to maximize their advantages for the diversity of more challenging biological utilization. Herein, a thiol-mediated multifunctional phospholipid ligand was designed while disclosing a zwitterionic nature to AuNPs. The ligand was synthesized by attachment to two bidentate lipoic acid (LA) anchor groups and incorporation of a zwitterionic phosphatidylcholine (PC) group, allowing for excellent hydrophilicity. As demonstrated through ultraviolet–visible spectroscopy, appropriate 7 nm diameter AuNPs modified with a 1,2-dilipoyl-sn-glycero-3-phosphorylcholine (di-LA-PC) compact ligand exhibited the best colloidal stability in a high NaCl concentration of up to 217 mM, different temperatures, and a wide range of pH values from 3 to 11 when compared to the traditional surfactants or thiol-contained amino acid surface modification cases. These AuNPs are also stable without specific interaction to positively/negatively charged proteins, possibly leading to prolonged blood circulation after in vivo administration. Moreover, much more resistance to ligand competition of dithiothreitol was found than other thiol-coated AuNPs, which further highlighted their affinity in an aqueous system. Biocompatibility of the zwitterionic ligand di-LA-PC-modified AuNPs was finally evaluated by hemolysis and cytotoxicity tests. Cumulatively, the remarkable stability and biocompatibility of AuNPs, multicoordinated with a di-LA-PC ligand, potentially motivated them as a practical alternative for surface tailoring in biotechnology.
中文翻译:

硫醇介导的多齿磷酸胆碱作为两性离子配体,用于稳定生物相容性金纳米颗粒。
金纳米颗粒(AuNPs)在生物医学中的日益广泛的应用要求对表面修饰和稳定性进行广泛的研究,以最大程度地发挥其在更具挑战性的生物利用方面的优势。在此,设计了硫醇介导的多功能磷脂配体,同时向AuNP公开了两性离子性质。通过连接到两个双齿硫辛酸(LA)锚定基团并结合两性离子磷脂酰胆碱(PC)基团来合成配体,从而获得出色的亲水性。如紫外可见光谱所证明的那样,用1,2-二脂基-sn修饰的合适的直径为7 nm的AuNPs与传统甘油酯相比,-glycero-3-phosphorylcholine(di-LA-PC)紧凑型配体在最高217 mM的高NaCl浓度,不同的温度以及3至11的宽pH值范围内均表现出最佳的胶体稳定性。表面活性剂或含巯基的氨基酸表面修饰的情况。这些AuNPs也很稳定,不会与带正电/带负电的蛋白质发生特异性相互作用,可能导致体内血液循环延长行政。此外,发现二硫苏糖醇对配体竞争的抵抗力比其他硫醇包被的AuNPs强,这进一步突出了它们在水性体系中的亲和力。两性离子配体di-LA-PC修饰的AuNPs的生物相容性最终通过溶血和细胞毒性测试进行了评估。累积地,与di-LA-PC配体多配位的AuNPs出色的稳定性和生物相容性,有可能促使它们成为生物技术中表面修饰的一种实用替代方法。
更新日期:2019-09-26
中文翻译:

硫醇介导的多齿磷酸胆碱作为两性离子配体,用于稳定生物相容性金纳米颗粒。
金纳米颗粒(AuNPs)在生物医学中的日益广泛的应用要求对表面修饰和稳定性进行广泛的研究,以最大程度地发挥其在更具挑战性的生物利用方面的优势。在此,设计了硫醇介导的多功能磷脂配体,同时向AuNP公开了两性离子性质。通过连接到两个双齿硫辛酸(LA)锚定基团并结合两性离子磷脂酰胆碱(PC)基团来合成配体,从而获得出色的亲水性。如紫外可见光谱所证明的那样,用1,2-二脂基-sn修饰的合适的直径为7 nm的AuNPs与传统甘油酯相比,-glycero-3-phosphorylcholine(di-LA-PC)紧凑型配体在最高217 mM的高NaCl浓度,不同的温度以及3至11的宽pH值范围内均表现出最佳的胶体稳定性。表面活性剂或含巯基的氨基酸表面修饰的情况。这些AuNPs也很稳定,不会与带正电/带负电的蛋白质发生特异性相互作用,可能导致体内血液循环延长行政。此外,发现二硫苏糖醇对配体竞争的抵抗力比其他硫醇包被的AuNPs强,这进一步突出了它们在水性体系中的亲和力。两性离子配体di-LA-PC修饰的AuNPs的生物相容性最终通过溶血和细胞毒性测试进行了评估。累积地,与di-LA-PC配体多配位的AuNPs出色的稳定性和生物相容性,有可能促使它们成为生物技术中表面修饰的一种实用替代方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号