当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural Basis for Achieving GSK-3β Inhibition with High Potency, Selectivity, and Brain Exposure for Positron Emission Tomography Imaging and Drug Discovery.
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2019-10-21 , DOI: 10.1021/acs.jmedchem.9b01030 Vadim Bernard-Gauthier 1, 2, 3 , Andrew V Mossine 4 , Ashley Knight 1, 2, 5 , Debasis Patnaik 6 , Wen-Ning Zhao 6 , Chialin Cheng 6 , Hema S Krishnan 3 , Lucius L Xuan 6 , Peter S Chindavong 6 , Surya A Reis 6 , Jinshan Michael Chen 7 , Xia Shao 4 , Jenelle Stauff 4 , Janna Arteaga 4 , Phillip Sherman 4 , Nicolas Salem 8 , David Bonsall 9 , Brenda Amaral 8 , Cassis Varlow 1 , Lisa Wells 9 , Laurent Martarello 8 , Shil Patel 5 , Steven H Liang 3 , Ravi G Kurumbail 7 , Stephen J Haggarty 6 , Peter J H Scott 4, 10 , Neil Vasdev 1, 2, 3
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2019-10-21 , DOI: 10.1021/acs.jmedchem.9b01030 Vadim Bernard-Gauthier 1, 2, 3 , Andrew V Mossine 4 , Ashley Knight 1, 2, 5 , Debasis Patnaik 6 , Wen-Ning Zhao 6 , Chialin Cheng 6 , Hema S Krishnan 3 , Lucius L Xuan 6 , Peter S Chindavong 6 , Surya A Reis 6 , Jinshan Michael Chen 7 , Xia Shao 4 , Jenelle Stauff 4 , Janna Arteaga 4 , Phillip Sherman 4 , Nicolas Salem 8 , David Bonsall 9 , Brenda Amaral 8 , Cassis Varlow 1 , Lisa Wells 9 , Laurent Martarello 8 , Shil Patel 5 , Steven H Liang 3 , Ravi G Kurumbail 7 , Stephen J Haggarty 6 , Peter J H Scott 4, 10 , Neil Vasdev 1, 2, 3
Affiliation
|
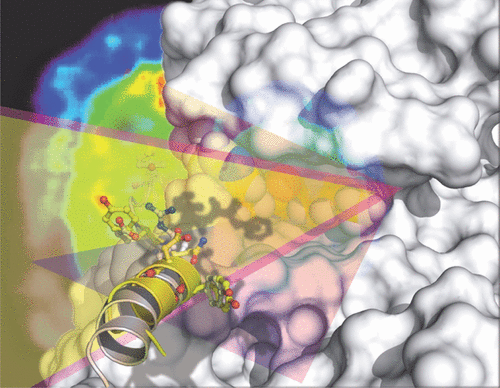
Using structure-guided design, several cell based assays, and microdosed positron emission tomography (PET) imaging, we identified a series of highly potent, selective, and brain-penetrant oxazole-4-carboxamide-based inhibitors of glycogen synthase kinase-3 (GSK-3). An isotopologue of our first-generation lead, [3H]PF-367, demonstrates selective and specific target engagement in vitro, irrespective of the activation state. We discovered substantial ubiquitous GSK-3-specific radioligand binding in Tg2576 Alzheimer’s disease (AD), suggesting application for these compounds in AD diagnosis and identified [11C]OCM-44 as our lead GSK-3 radiotracer, with optimized brain uptake by PET imaging in nonhuman primates. GSK-3β-isozyme selectivity was assessed to reveal OCM-51, the most potent (IC50 = 0.030 nM) and selective (>10-fold GSK-3β/GSK-3α) GSK-3β inhibitor known to date. Inhibition of CRMP2T514 and tau phosphorylation, as well as favorable therapeutic window against WNT/β-catenin signaling activation, was observed in cells.
中文翻译:

以高效力、选择性和脑暴露实现正电子发射断层扫描成像和药物发现的 GSK-3β 抑制的结构基础。
使用结构引导设计、多种基于细胞的测定和微剂量正电子发射断层扫描 (PET) 成像,我们鉴定了一系列高效、选择性和脑渗透性基于恶唑-4-甲酰胺的糖原合酶激酶 3 抑制剂。葛兰素史克-3)。我们的第一代先导化合物 [ 3 H]PF-367 的同位素体在体外表现出选择性和特异性的靶标参与,而与激活状态无关。我们在 Tg2576 阿尔茨海默病 (AD) 中发现了大量普遍存在的 GSK-3 特异性放射性配体结合,建议这些化合物在 AD 诊断中的应用,并确定 [ 11 C]OCM-44 作为我们的主要 GSK-3 放射性示踪剂,并通过 PET 优化大脑摄取非人类灵长类动物的成像。评估 GSK-3β-同工酶选择性以揭示 OCM-51,这是迄今为止已知的最有效(IC 50 = 0.030 nM)和选择性(>10 倍 GSK-3β/GSK-3α)GSK-3β 抑制剂。在细胞中观察到 CRMP2 T514和 tau 磷酸化的抑制,以及针对 WNT/β-连环蛋白信号激活的有利治疗窗口。
更新日期:2019-10-21
中文翻译:

以高效力、选择性和脑暴露实现正电子发射断层扫描成像和药物发现的 GSK-3β 抑制的结构基础。
使用结构引导设计、多种基于细胞的测定和微剂量正电子发射断层扫描 (PET) 成像,我们鉴定了一系列高效、选择性和脑渗透性基于恶唑-4-甲酰胺的糖原合酶激酶 3 抑制剂。葛兰素史克-3)。我们的第一代先导化合物 [ 3 H]PF-367 的同位素体在体外表现出选择性和特异性的靶标参与,而与激活状态无关。我们在 Tg2576 阿尔茨海默病 (AD) 中发现了大量普遍存在的 GSK-3 特异性放射性配体结合,建议这些化合物在 AD 诊断中的应用,并确定 [ 11 C]OCM-44 作为我们的主要 GSK-3 放射性示踪剂,并通过 PET 优化大脑摄取非人类灵长类动物的成像。评估 GSK-3β-同工酶选择性以揭示 OCM-51,这是迄今为止已知的最有效(IC 50 = 0.030 nM)和选择性(>10 倍 GSK-3β/GSK-3α)GSK-3β 抑制剂。在细胞中观察到 CRMP2 T514和 tau 磷酸化的抑制,以及针对 WNT/β-连环蛋白信号激活的有利治疗窗口。

































 京公网安备 11010802027423号
京公网安备 11010802027423号