当前位置:
X-MOL 学术
›
Theranostics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chlorogenic acid effectively treats cancers through induction of cancer cell differentiation.
Theranostics ( IF 12.4 ) Pub Date : 2019-09-19 , DOI: 10.7150/thno.34674 Shuai Huang 1, 2 , Lu-Lu Wang 1 , Ni-Na Xue 1 , Cong Li 1 , Hui-Hui Guo 1 , Tian-Kun Ren 1 , Yun Zhan 1 , Wen-Bing Li 3 , Jie Zhang 4 , Xiao-Guang Chen 1 , Yan-Xing Han 1 , Jin-Lan Zhang 1 , Jian-Dong Jiang 1, 2
Theranostics ( IF 12.4 ) Pub Date : 2019-09-19 , DOI: 10.7150/thno.34674 Shuai Huang 1, 2 , Lu-Lu Wang 1 , Ni-Na Xue 1 , Cong Li 1 , Hui-Hui Guo 1 , Tian-Kun Ren 1 , Yun Zhan 1 , Wen-Bing Li 3 , Jie Zhang 4 , Xiao-Guang Chen 1 , Yan-Xing Han 1 , Jin-Lan Zhang 1 , Jian-Dong Jiang 1, 2
Affiliation
|
RATIONALE
Inducing cancer differentiation is a promising approach to treat cancer. Here, we identified chlorogenic acid (CA), a potential differentiation inducer, for cancer therapy, and elucidated the molecular mechanisms underlying its differentiation-inducing effects on cancer cells.
METHODS
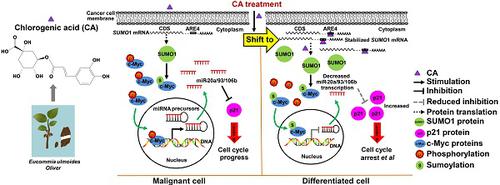
Cancer cell differentiation was investigated by measuring malignant behavior, including growth rate, invasion/migration, morphological change, maturation, and ATP production. Gene expression was analyzed by microarray analysis, qRT-PCR, and protein measurement, and molecular biology techniques were employed for mechanistic studies. LC/MS analysis was the method of choice for chemical detection. Finally, the anticancer effect of CA was evaluated both in vitro and in vivo. Results: Cancer cells treated with CA showed reduced proliferation rate, migration/invasion ability, and mitochondrial ATP production. Treating cancer cells with CA resulted in elevated SUMO1 expression through acting on its 3'UTR and stabilizing the mRNA. The increased SUMO1 caused c-Myc sumoylation, miR-17 family downregulation, and p21 upregulation leading to G0/G1 arrest and maturation phenotype. CA altered the expression of differentiation-related genes in cancer cells but not in normal cells. It inhibited hepatoma and lung cancer growth in tumor-bearing mice and prevented new tumor development in naïve mice. In glioma cells, CA increased expression of specific differentiation biomarkers Tuj1 and GFAP inducing differentiation and reducing sphere formation. The therapeutic efficacy of CA in glioma cells was comparable to that of temozolomide. CA was detectable both in the blood and brain when administered intraperitoneally in animals. Most importantly, CA was safe even at very high doses.
CONCLUSION
CA might be a safe and effective differentiation-inducer for cancer therapy. "Educating" cancer cells to differentiate, rather than killing them, could be a novel therapeutic strategy for cancer.
中文翻译:

绿原酸通过诱导癌细胞分化有效地治疗癌症。
理由诱导癌症分化是治疗癌症的有前途的方法。在这里,我们确定了绿原酸(CA),一种潜在的分化诱导剂,用于癌症治疗,并阐明了其诱导分化作用对癌细胞的分子机制。方法通过测量恶性行为,包括生长速度,侵袭/迁移,形态变化,成熟度和ATP产生来研究癌细胞的分化。通过微阵列分析,qRT-PCR和蛋白质测量分析基因表达,并采用分子生物学技术进行机理研究。LC / MS分析是化学检测的首选方法。最后,在体外和体内均评估了CA的抗癌作用。结果:CA处理的癌细胞显示出降低的增殖速率,迁移/入侵能力和线粒体ATP产生。用CA处理癌细胞可通过作用于其3'UTR并使mRNA稳定来提高SUMO1的表达。增加的SUMO1导致c-Myc sumoylation,miR-17家族下调和p21上调,导致G0 / G1阻滞和成熟表型。CA改变了癌细胞中分化相关基因的表达,但没有改变正常细胞中的分化相关基因的表达。它可以抑制荷瘤小鼠的肝癌和肺癌的生长,并可以防止幼稚小鼠出现新的肿瘤。在神经胶质瘤细胞中,CA增加了特定分化生物标志物Tuj1和GFAP的表达,从而诱导分化并减少了球的形成。CA在神经胶质瘤细胞中的治疗功效与替莫唑胺相当。在动物腹膜内给药时,在血液和大脑中都可以检测到CA。最重要的是,即使在非常高的剂量下,CA也是安全的。结论CA可能是一种安全有效的癌症分化诱导剂。“教育”癌细胞以分化而不是杀死它们,可能是一种新颖的癌症治疗策略。
更新日期:2019-09-20
中文翻译:

绿原酸通过诱导癌细胞分化有效地治疗癌症。
理由诱导癌症分化是治疗癌症的有前途的方法。在这里,我们确定了绿原酸(CA),一种潜在的分化诱导剂,用于癌症治疗,并阐明了其诱导分化作用对癌细胞的分子机制。方法通过测量恶性行为,包括生长速度,侵袭/迁移,形态变化,成熟度和ATP产生来研究癌细胞的分化。通过微阵列分析,qRT-PCR和蛋白质测量分析基因表达,并采用分子生物学技术进行机理研究。LC / MS分析是化学检测的首选方法。最后,在体外和体内均评估了CA的抗癌作用。结果:CA处理的癌细胞显示出降低的增殖速率,迁移/入侵能力和线粒体ATP产生。用CA处理癌细胞可通过作用于其3'UTR并使mRNA稳定来提高SUMO1的表达。增加的SUMO1导致c-Myc sumoylation,miR-17家族下调和p21上调,导致G0 / G1阻滞和成熟表型。CA改变了癌细胞中分化相关基因的表达,但没有改变正常细胞中的分化相关基因的表达。它可以抑制荷瘤小鼠的肝癌和肺癌的生长,并可以防止幼稚小鼠出现新的肿瘤。在神经胶质瘤细胞中,CA增加了特定分化生物标志物Tuj1和GFAP的表达,从而诱导分化并减少了球的形成。CA在神经胶质瘤细胞中的治疗功效与替莫唑胺相当。在动物腹膜内给药时,在血液和大脑中都可以检测到CA。最重要的是,即使在非常高的剂量下,CA也是安全的。结论CA可能是一种安全有效的癌症分化诱导剂。“教育”癌细胞以分化而不是杀死它们,可能是一种新颖的癌症治疗策略。






























 京公网安备 11010802027423号
京公网安备 11010802027423号