当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Feasibility of Electrochemical Ammonia Synthesis in Molten LiCl-KCl Eutectics.
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-10-15 , DOI: 10.1002/anie.201909831 Ian J McPherson 1 , Tim Sudmeier 1 , Joshua P Fellowes 1 , Ian Wilkinson 2 , Tim Hughes 2 , S C Edman Tsang 1
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2019-10-15 , DOI: 10.1002/anie.201909831 Ian J McPherson 1 , Tim Sudmeier 1 , Joshua P Fellowes 1 , Ian Wilkinson 2 , Tim Hughes 2 , S C Edman Tsang 1
Affiliation
|
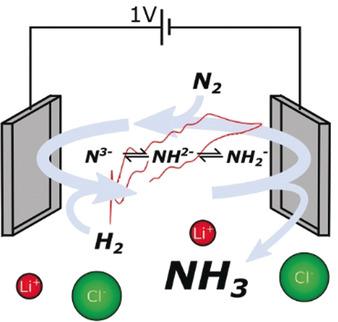
Molten LiCl and related eutectic electrolytes are known to permit direct electrochemical reduction of N2 to N3- with high efficiency. It had been proposed that this could be coupled with H2 oxidation in an electrolytic cell to produce NH3 at ambient pressure. Here, this proposal is tested in a LiCl-KCl-Li3 N cell and is found not to be the case, as the previous assumption of the direct electrochemical oxidation of N3- to NH3 is grossly over-simplified. We find that Li3 N added to the molten electrolyte promotes the spontaneous and simultaneous chemical disproportionation of H2 (H oxidation state 0) into H- (H oxidation state -1) and H+ in the form of NH2- /NH2 - /NH3 (H oxidation state +1) in the absence of applied current, resulting in non-Faradaic release of NH3 . It is further observed that NH2- and NH2 - possess their own redox chemistry. However, these spontaneous reactions allow us to propose an alternative, truly catalytic cycle. By adding LiH, rather than Li3 N, N2 can be reduced to N3- while stoichiometric amounts of H- are oxidised to H2 . The H2 can then react spontaneously with N3- to form NH3 , regenerating H- and closing the catalytic cycle. Initial tests show a peak NH3 synthesis rate of 2.4×10-8 mol cm-2 s-1 at a maximum current efficiency of 4.2 %. Isotopic labelling with 15 N2 confirms the resulting NH3 is from catalytic N2 reduction.
中文翻译:

LiCl-KCl共熔物中电化学合成氨的可行性。
已知熔融的LiCl和相关的低共熔电解质允许将N 2直接电化学高效还原为N 3。已经提出,这可以与电解池中的H 2氧化结合以在环境压力下产生NH 3。在此,该提议在LiCl-KCl-Li3 N电池中进行了测试,但事实并非如此,因为以前将N3-直接电化学氧化为NH3的假设被大大简化了。我们发现,添加到熔融电解质中的Li3 N促进了H2(H氧化态0)自发同时发生化学歧化,转变为H2-(H氧化态-1)和H +,其形式为NH2- / NH2--/ NH3(H在没有施加电流的情况下发生氧化态+1),导致非法拉第释放NH3。进一步观察到,NH 2-和NH 2-具有它们自己的氧化还原化学。但是,这些自发反应使我们可以提出另一种真正的催化循环。通过添加LiH而不是Li3 N,N2可以还原为N3-,而化学计量的H-被氧化为H2。然后,H2可以与N3-自发反应形成NH3,再生H-并关闭催化循环。初步测试显示,在4.2%的最大电流效率下,NH3的合成峰值速率为2.4×10-8 mol cm-2 s-1。用15 N2进行同位素标记,确认生成的NH3来自催化还原的N2。初步测试显示,在4.2%的最大电流效率下,NH3的合成峰值速率为2.4×10-8 mol cm-2 s-1。用15 N2进行同位素标记,确认生成的NH3来自催化还原的N2。初步测试显示,在4.2%的最大电流效率下,NH3的合成峰值速率为2.4×10-8 mol cm-2 s-1。用15 N2进行同位素标记,确认生成的NH3来自催化还原的N2。
更新日期:2019-10-16
中文翻译:

LiCl-KCl共熔物中电化学合成氨的可行性。
已知熔融的LiCl和相关的低共熔电解质允许将N 2直接电化学高效还原为N 3。已经提出,这可以与电解池中的H 2氧化结合以在环境压力下产生NH 3。在此,该提议在LiCl-KCl-Li3 N电池中进行了测试,但事实并非如此,因为以前将N3-直接电化学氧化为NH3的假设被大大简化了。我们发现,添加到熔融电解质中的Li3 N促进了H2(H氧化态0)自发同时发生化学歧化,转变为H2-(H氧化态-1)和H +,其形式为NH2- / NH2--/ NH3(H在没有施加电流的情况下发生氧化态+1),导致非法拉第释放NH3。进一步观察到,NH 2-和NH 2-具有它们自己的氧化还原化学。但是,这些自发反应使我们可以提出另一种真正的催化循环。通过添加LiH而不是Li3 N,N2可以还原为N3-,而化学计量的H-被氧化为H2。然后,H2可以与N3-自发反应形成NH3,再生H-并关闭催化循环。初步测试显示,在4.2%的最大电流效率下,NH3的合成峰值速率为2.4×10-8 mol cm-2 s-1。用15 N2进行同位素标记,确认生成的NH3来自催化还原的N2。初步测试显示,在4.2%的最大电流效率下,NH3的合成峰值速率为2.4×10-8 mol cm-2 s-1。用15 N2进行同位素标记,确认生成的NH3来自催化还原的N2。初步测试显示,在4.2%的最大电流效率下,NH3的合成峰值速率为2.4×10-8 mol cm-2 s-1。用15 N2进行同位素标记,确认生成的NH3来自催化还原的N2。






























 京公网安备 11010802027423号
京公网安备 11010802027423号