当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the Role of Metal and Molecular Structure on the Electrocatalytic Hydrogenation of Oxygenated Organic Compounds
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-10-04 , DOI: 10.1021/acscatal.9b02921
Juan A. Lopez-Ruiz 1 , Evan Andrews 1 , Sneha A. Akhade 1, 2 , Mal-Soon Lee 1 , Katherine Koh 1 , Udishnu Sanyal 1 , Simuck F. Yuk 1 , Abhijeet J. Karkamkar 1 , Miroslaw A. Derewinski 1 , Johnathan Holladay 1 , Vassiliki-Alexandra Glezakou 1 , Roger Rousseau 1 , Oliver Y. Gutiérrez 1 , Jamie D. Holladay 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-10-04 , DOI: 10.1021/acscatal.9b02921
Juan A. Lopez-Ruiz 1 , Evan Andrews 1 , Sneha A. Akhade 1, 2 , Mal-Soon Lee 1 , Katherine Koh 1 , Udishnu Sanyal 1 , Simuck F. Yuk 1 , Abhijeet J. Karkamkar 1 , Miroslaw A. Derewinski 1 , Johnathan Holladay 1 , Vassiliki-Alexandra Glezakou 1 , Roger Rousseau 1 , Oliver Y. Gutiérrez 1 , Jamie D. Holladay 1
Affiliation
|
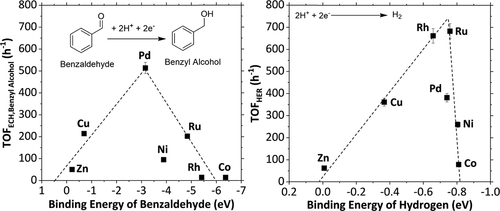
Electrocatalytic hydrogenation is increasingly studied as an alternative to integrate the use of recycled carbon feedstocks with renewable energy sources. However, the abundant empiric observations available have not been correlated with fundamental properties of substrates and catalysts. In this study, we investigated electrocatalytic hydrogenation of a homologues series of carboxylic acids, ketones, phenolics, and aldehydes on a variety of metals (Pd, Rh, Ru, Cu, Ni, Zn, and Co). We found that the rates of carbonyl reduction in aldehydes correlate with the corresponding binding energies between the aldehydes and the metals according to the Sabatier principle. That is, the highest rates are obtained at intermediate binding energies. The rates of H2 evolution that occur in parallel to hydrogenation also correlate with the H-metal binding energies, following the same volcano-type behavior. Within the boundaries of this model (e.g., compounds reactive at room temperature and without important steric effects over the carbonyl group), the reported correlations help to explain the complex trends derived from the experimental observations, allowing for the correlation of rates with binding energies and the differentiation of mechanistic routes.
中文翻译:

了解金属和分子结构在氧化有机化合物电催化加氢中的作用
越来越多地研究电催化加氢作为将回收碳原料与可再生能源结合使用的替代方法。但是,可用的大量经验性观察与底物和催化剂的基本性能没有关系。在这项研究中,我们研究了在多种金属(Pd,Rh,Ru,Cu,Ni,Zn和Co)上羧酸,酮,酚醛和醛的同系物系列的电催化氢化反应。我们发现,根据Sabatier原理,醛中羰基的还原速率与醛与金属之间的相应结合能相关。即,在中间结合能处获得最高速率。H 2的比率遵循相同的火山类型行为,与氢化平行发生的析出也与H-金属的结合能相关。在该模型的范围内(例如,在室温下具有反应性且对羰基没有重要空间效应的化合物),所报道的相关性有助于解释从实验观察中得出的复杂趋势,从而使速率与结合能和机械路线的区别。
更新日期:2019-10-05
中文翻译:

了解金属和分子结构在氧化有机化合物电催化加氢中的作用
越来越多地研究电催化加氢作为将回收碳原料与可再生能源结合使用的替代方法。但是,可用的大量经验性观察与底物和催化剂的基本性能没有关系。在这项研究中,我们研究了在多种金属(Pd,Rh,Ru,Cu,Ni,Zn和Co)上羧酸,酮,酚醛和醛的同系物系列的电催化氢化反应。我们发现,根据Sabatier原理,醛中羰基的还原速率与醛与金属之间的相应结合能相关。即,在中间结合能处获得最高速率。H 2的比率遵循相同的火山类型行为,与氢化平行发生的析出也与H-金属的结合能相关。在该模型的范围内(例如,在室温下具有反应性且对羰基没有重要空间效应的化合物),所报道的相关性有助于解释从实验观察中得出的复杂趋势,从而使速率与结合能和机械路线的区别。

































 京公网安备 11010802027423号
京公网安备 11010802027423号