Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Architecture of Talin1 Reveals an Autoinhibition Mechanism.
Cell ( IF 45.5 ) Pub Date : 2019-09-19 , DOI: 10.1016/j.cell.2019.08.034 Dirk Dedden 1 , Stephanie Schumacher 1 , Charlotte F Kelley 1 , Martin Zacharias 2 , Christian Biertümpfel 1 , Reinhard Fässler 3 , Naoko Mizuno 1
Cell ( IF 45.5 ) Pub Date : 2019-09-19 , DOI: 10.1016/j.cell.2019.08.034 Dirk Dedden 1 , Stephanie Schumacher 1 , Charlotte F Kelley 1 , Martin Zacharias 2 , Christian Biertümpfel 1 , Reinhard Fässler 3 , Naoko Mizuno 1
Affiliation
|
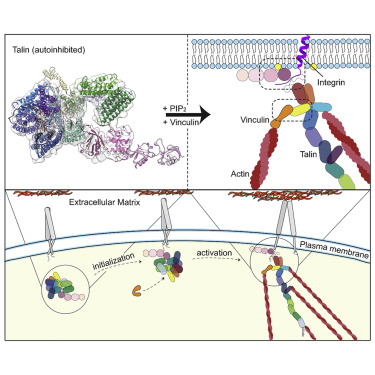
Focal adhesions (FAs) are protein machineries essential for cell adhesion, migration, and differentiation. Talin is an integrin-activating and tension-sensing FA component directly connecting integrins in the plasma membrane with the actomyosin cytoskeleton. To understand how talin function is regulated, we determined a cryoelectron microscopy (cryo-EM) structure of full-length talin1 revealing a two-way mode of autoinhibition. The actin-binding rod domains fold into a 15-nm globular arrangement that is interlocked by the integrin-binding FERM head. In turn, the rod domains R9 and R12 shield access of the FERM domain to integrin and the phospholipid PIP2 at the membrane. This mechanism likely ensures synchronous inhibition of integrin, membrane, and cytoskeleton binding. We also demonstrate that compacted talin1 reversibly unfolds to an ∼60-nm string-like conformation, revealing interaction sites for vinculin and actin. Our data explain how fast switching between active and inactive conformations of talin could regulate FA turnover, a process critical for cell adhesion and signaling.
中文翻译:

Talin1的体系结构揭示了一种自动抑制机制。
局灶性粘附(FAs)是细胞粘附,迁移和分化所必需的蛋白质机制。Talin是一种整合素激活和张力感应FA成分,直接将质膜中的整合素与放线菌素细胞骨架相连。为了了解塔林蛋白的功能是如何调节的,我们确定了全长塔林蛋白1的冷冻电子显微镜(cryo-EM)结构,揭示了一种自抑制的双向模式。肌动蛋白结合杆结构域折叠成15纳米球状排列,由整联蛋白结合FERM头互锁。继而,杆结构域R9和R12屏蔽FERM结构域对膜上整联蛋白和磷脂PIP2的进入。该机制可能确保同步抑制整联蛋白,膜和细胞骨架结合。我们还证明了紧密的talin1可逆地展开为约60 nm的弦状构象,揭示了纽蛋白和肌动蛋白的相互作用位点。我们的数据解释了在塔林蛋白有效构象和非活性构象之间的快速切换如何调节FA转换,FA转换对细胞粘附和信号传导至关重要。
更新日期:2019-09-30
中文翻译:

Talin1的体系结构揭示了一种自动抑制机制。
局灶性粘附(FAs)是细胞粘附,迁移和分化所必需的蛋白质机制。Talin是一种整合素激活和张力感应FA成分,直接将质膜中的整合素与放线菌素细胞骨架相连。为了了解塔林蛋白的功能是如何调节的,我们确定了全长塔林蛋白1的冷冻电子显微镜(cryo-EM)结构,揭示了一种自抑制的双向模式。肌动蛋白结合杆结构域折叠成15纳米球状排列,由整联蛋白结合FERM头互锁。继而,杆结构域R9和R12屏蔽FERM结构域对膜上整联蛋白和磷脂PIP2的进入。该机制可能确保同步抑制整联蛋白,膜和细胞骨架结合。我们还证明了紧密的talin1可逆地展开为约60 nm的弦状构象,揭示了纽蛋白和肌动蛋白的相互作用位点。我们的数据解释了在塔林蛋白有效构象和非活性构象之间的快速切换如何调节FA转换,FA转换对细胞粘附和信号传导至关重要。































 京公网安备 11010802027423号
京公网安备 11010802027423号