当前位置:
X-MOL 学术
›
ACS Sustain. Chem. Eng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interface Engineering of MoS2 for Electrocatalytic Performance Optimization for Hydrogen Generation via Urea Electrolysis
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-09-30 , DOI: 10.1021/acssuschemeng.9b03906 Zexing Wu 1 , Xuyun Guo 2 , Zhanhao Zhang 1 , Min Song 1 , Tiantian Jiao 3 , Ye Zhu 2 , Jie Wang 4 , Xien Liu 1
ACS Sustainable Chemistry & Engineering ( IF 7.1 ) Pub Date : 2019-09-30 , DOI: 10.1021/acssuschemeng.9b03906 Zexing Wu 1 , Xuyun Guo 2 , Zhanhao Zhang 1 , Min Song 1 , Tiantian Jiao 3 , Ye Zhu 2 , Jie Wang 4 , Xien Liu 1
Affiliation
|
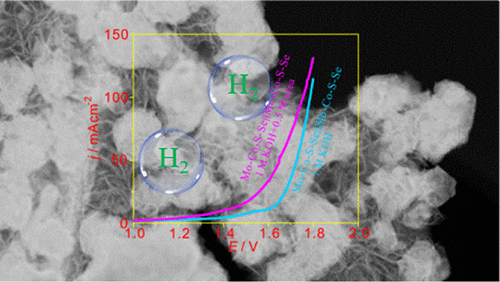
Developing highly efficient and low-cost nonprecious electrocatalysts for hydrogen evolution reaction (HER) has a pivotal impact on the emergence of hydrogen energy. Herein, quaternary electrocatalyst characterized by abundant interfaces supported on carbon cloth (denoted as Mo–Co–S–Se/CC) is designed through a facile solvothermal and post-low-temperature selenylation process, which delivers excellent catalytic performances in HER, oxygen evolution reaction (OER), and urea oxidation reaction (UOR) in alkaline electrolyte. Benefiting from the rich interfaces, the designed catalyst delivers current densities of 10 and 100 mA cm–2 with low overpotentials of 58 and 167 mV, respectively, and small Tafel slope of 84 mV dec–1 for HER. For the anodic OER, only 350 mV overpotential is needed to drive 100 mA cm–2 in 1 M KOH solution. Moreover, Mo–Co–S–Se/CC also presents remarkable catalytic activity for UOR in 1 M KOH solution, which provides another way to substitute the sluggish OER to reduce the cost of hydrogen production. As a proof of concept, overall water-splitting tests are measured with Mo–Co–S–Se/CC as both anode and cathode, respectively, in 1 M KOH solution with 0.5 M urea; only 1.4 V is required to drive 10 mA cm–2, much lower than that for urea-free electrolyte with 1.62 V.
中文翻译:

MoS 2的界面工程,用于优化尿素电解制氢的电催化性能
开发用于氢气析出反应(HER)的高效,低成本非贵金属电催化剂对氢能的产生具有至关重要的影响。在此,以轻质的溶剂热和低温后硒化反应工艺设计了以碳布为载体的界面丰富的四元电催化剂(表示为Mo–Co–S–Se / CC),在HER,氧气释放方面具有出色的催化性能。反应(OER)和尿素氧化反应(UOR)在碱性电解液中。得益于丰富的界面,设计的催化剂可提供10和100 mA cm –2的电流密度,分别具有58和167 mV的低过电势以及84 mV dec –1的小Tafel斜率。为了她。对于阳极OER,在1 M KOH溶液中驱动100 mA cm –2时仅需要350 mV的超电势。此外,Mo-Co-S-Se / CC还对1M KOH溶液中的UOR表现出显着的催化活性,这为替代缓慢的OER提供了另一种方式来降低制氢成本。作为概念证明,在1 M KOH溶液和0.5 M尿素中,分别使用Mo–Co–S–Se / CC作为阳极和阴极来测量总的水分解试验。驱动10 mA cm –2只需1.4 V ,远低于1.62 V的无尿素电解液。
更新日期:2019-09-30
中文翻译:

MoS 2的界面工程,用于优化尿素电解制氢的电催化性能
开发用于氢气析出反应(HER)的高效,低成本非贵金属电催化剂对氢能的产生具有至关重要的影响。在此,以轻质的溶剂热和低温后硒化反应工艺设计了以碳布为载体的界面丰富的四元电催化剂(表示为Mo–Co–S–Se / CC),在HER,氧气释放方面具有出色的催化性能。反应(OER)和尿素氧化反应(UOR)在碱性电解液中。得益于丰富的界面,设计的催化剂可提供10和100 mA cm –2的电流密度,分别具有58和167 mV的低过电势以及84 mV dec –1的小Tafel斜率。为了她。对于阳极OER,在1 M KOH溶液中驱动100 mA cm –2时仅需要350 mV的超电势。此外,Mo-Co-S-Se / CC还对1M KOH溶液中的UOR表现出显着的催化活性,这为替代缓慢的OER提供了另一种方式来降低制氢成本。作为概念证明,在1 M KOH溶液和0.5 M尿素中,分别使用Mo–Co–S–Se / CC作为阳极和阴极来测量总的水分解试验。驱动10 mA cm –2只需1.4 V ,远低于1.62 V的无尿素电解液。






























 京公网安备 11010802027423号
京公网安备 11010802027423号