Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrical and synaptic integration of glioma into neural circuits
Nature ( IF 50.5 ) Pub Date : 2019-09-18 , DOI: 10.1038/s41586-019-1563-y
Humsa S Venkatesh 1 , Wade Morishita 2, 3 , Anna C Geraghty 1 , Dana Silverbush 4, 5, 6 , Shawn M Gillespie 1 , Marlene Arzt 1 , Lydia T Tam 1 , Cedric Espenel 7 , Anitha Ponnuswami 1 , Lijun Ni 1 , Pamelyn J Woo 1 , Kathryn R Taylor 1 , Amit Agarwal 8, 9 , Aviv Regev 5, 6, 10 , David Brang 11 , Hannes Vogel 1, 12, 13 , Shawn Hervey-Jumper 14 , Dwight E Bergles 8 , Mario L Suvà 4, 5, 6 , Robert C Malenka 2, 3 , Michelle Monje 1, 2, 12, 13, 15
Nature ( IF 50.5 ) Pub Date : 2019-09-18 , DOI: 10.1038/s41586-019-1563-y
Humsa S Venkatesh 1 , Wade Morishita 2, 3 , Anna C Geraghty 1 , Dana Silverbush 4, 5, 6 , Shawn M Gillespie 1 , Marlene Arzt 1 , Lydia T Tam 1 , Cedric Espenel 7 , Anitha Ponnuswami 1 , Lijun Ni 1 , Pamelyn J Woo 1 , Kathryn R Taylor 1 , Amit Agarwal 8, 9 , Aviv Regev 5, 6, 10 , David Brang 11 , Hannes Vogel 1, 12, 13 , Shawn Hervey-Jumper 14 , Dwight E Bergles 8 , Mario L Suvà 4, 5, 6 , Robert C Malenka 2, 3 , Michelle Monje 1, 2, 12, 13, 15
Affiliation
|
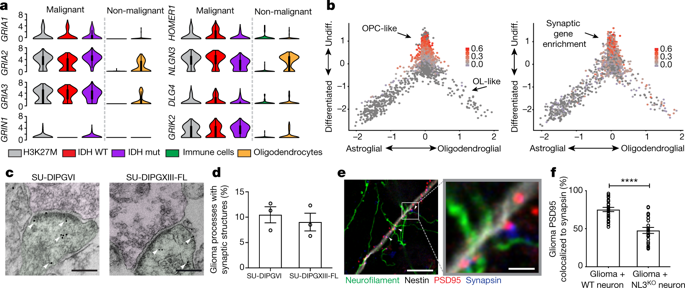
High-grade gliomas are lethal brain cancers whose progression is robustly regulated by neuronal activity. Activity-regulated release of growth factors promotes glioma growth, but this alone is insufficient to explain the effect that neuronal activity exerts on glioma progression. Here we show that neuron and glioma interactions include electrochemical communication through bona fide AMPA receptor-dependent neuron–glioma synapses. Neuronal activity also evokes non-synaptic activity-dependent potassium currents that are amplified by gap junction-mediated tumour interconnections, forming an electrically coupled network. Depolarization of glioma membranes assessed by in vivo optogenetics promotes proliferation, whereas pharmacologically or genetically blocking electrochemical signalling inhibits the growth of glioma xenografts and extends mouse survival. Emphasizing the positive feedback mechanisms by which gliomas increase neuronal excitability and thus activity-regulated glioma growth, human intraoperative electrocorticography demonstrates increased cortical excitability in the glioma-infiltrated brain. Together, these findings indicate that synaptic and electrical integration into neural circuits promotes glioma progression.Neurons form synapses onto glioma cells, and depolarization of glioma membranes promotes glioma growth in vivo, whereas blocking electrochemical signalling blocks tumour growth.
中文翻译:

神经胶质瘤与神经回路的电和突触整合
高级别胶质瘤是致命的脑癌,其进展受到神经元活动的强烈调节。生长因子的活性调节释放促进了神经胶质瘤的生长,但仅此一项不足以解释神经元活动对神经胶质瘤进展的影响。在这里,我们表明神经元和神经胶质瘤的相互作用包括通过真正的 AMPA 受体依赖性神经元 - 神经胶质瘤突触的电化学通信。神经元活动还引起非突触活动依赖性钾电流,这些电流被间隙连接介导的肿瘤互连放大,形成电耦合网络。通过体内光遗传学评估的神经胶质瘤膜的去极化促进增殖,而药理学或基因阻断电化学信号传导抑制胶质瘤异种移植物的生长并延长小鼠的存活率。强调神经胶质瘤增加神经元兴奋性并因此活动调节的神经胶质瘤生长的正反馈机制,人类术中皮层电图显示胶质瘤浸润的大脑中皮质兴奋性增加。总之,这些发现表明突触和电整合到神经回路中促进了神经胶质瘤的进展。神经元在神经胶质瘤细胞上形成突触,神经胶质瘤膜的去极化促进了体内神经胶质瘤的生长,而阻断电化学信号传导会阻止肿瘤的生长。人类术中皮层电图显示胶质瘤浸润大脑的皮层兴奋性增加。总之,这些发现表明突触和电整合到神经回路中促进了神经胶质瘤的进展。神经元在神经胶质瘤细胞上形成突触,神经胶质瘤膜的去极化促进了体内神经胶质瘤的生长,而阻断电化学信号传导会阻止肿瘤的生长。人类术中皮层电图显示胶质瘤浸润大脑的皮层兴奋性增加。总之,这些发现表明突触和电整合到神经回路中促进了神经胶质瘤的进展。神经元在神经胶质瘤细胞上形成突触,神经胶质瘤膜的去极化促进了体内神经胶质瘤的生长,而阻断电化学信号传导会阻止肿瘤的生长。
更新日期:2019-09-18
中文翻译:

神经胶质瘤与神经回路的电和突触整合
高级别胶质瘤是致命的脑癌,其进展受到神经元活动的强烈调节。生长因子的活性调节释放促进了神经胶质瘤的生长,但仅此一项不足以解释神经元活动对神经胶质瘤进展的影响。在这里,我们表明神经元和神经胶质瘤的相互作用包括通过真正的 AMPA 受体依赖性神经元 - 神经胶质瘤突触的电化学通信。神经元活动还引起非突触活动依赖性钾电流,这些电流被间隙连接介导的肿瘤互连放大,形成电耦合网络。通过体内光遗传学评估的神经胶质瘤膜的去极化促进增殖,而药理学或基因阻断电化学信号传导抑制胶质瘤异种移植物的生长并延长小鼠的存活率。强调神经胶质瘤增加神经元兴奋性并因此活动调节的神经胶质瘤生长的正反馈机制,人类术中皮层电图显示胶质瘤浸润的大脑中皮质兴奋性增加。总之,这些发现表明突触和电整合到神经回路中促进了神经胶质瘤的进展。神经元在神经胶质瘤细胞上形成突触,神经胶质瘤膜的去极化促进了体内神经胶质瘤的生长,而阻断电化学信号传导会阻止肿瘤的生长。人类术中皮层电图显示胶质瘤浸润大脑的皮层兴奋性增加。总之,这些发现表明突触和电整合到神经回路中促进了神经胶质瘤的进展。神经元在神经胶质瘤细胞上形成突触,神经胶质瘤膜的去极化促进了体内神经胶质瘤的生长,而阻断电化学信号传导会阻止肿瘤的生长。人类术中皮层电图显示胶质瘤浸润大脑的皮层兴奋性增加。总之,这些发现表明突触和电整合到神经回路中促进了神经胶质瘤的进展。神经元在神经胶质瘤细胞上形成突触,神经胶质瘤膜的去极化促进了体内神经胶质瘤的生长,而阻断电化学信号传导会阻止肿瘤的生长。







































 京公网安备 11010802027423号
京公网安备 11010802027423号