Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An oligomeric state-dependent switch in the ER enzyme FICD regulates AMPylation and deAMPylation of BiP.
The EMBO Journal ( IF 9.4 ) Pub Date : 2019-09-18 , DOI: 10.15252/embj.2019102177 Luke A Perera 1 , Claudia Rato 1 , Yahui Yan 1 , Lisa Neidhardt 1 , Stephen H McLaughlin 2 , Randy J Read 1 , Steffen Preissler 1 , David Ron 1
The EMBO Journal ( IF 9.4 ) Pub Date : 2019-09-18 , DOI: 10.15252/embj.2019102177 Luke A Perera 1 , Claudia Rato 1 , Yahui Yan 1 , Lisa Neidhardt 1 , Stephen H McLaughlin 2 , Randy J Read 1 , Steffen Preissler 1 , David Ron 1
Affiliation
|
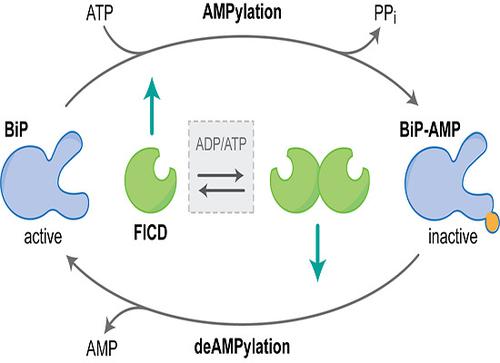
AMPylation is an inactivating modification that alters the activity of the major endoplasmic reticulum (ER) chaperone BiP to match the burden of unfolded proteins. A single ER-localised Fic protein, FICD (HYPE), catalyses both AMPylation and deAMPylation of BiP. However, the basis for the switch in FICD's activity is unknown. We report on the transition of FICD from a dimeric enzyme, that deAMPylates BiP, to a monomer with potent AMPylation activity. Mutations in the dimer interface, or of residues along an inhibitory pathway linking the dimer interface to the enzyme's active site, favour BiP AMPylation in vitro and in cells. Mechanistically, monomerisation relieves a repressive effect allosterically propagated from the dimer interface to the inhibitory Glu234, thereby permitting AMPylation-competent binding of MgATP. Moreover, a reciprocal signal, propagated from the nucleotide-binding site, provides a mechanism for coupling the oligomeric state and enzymatic activity of FICD to the energy status of the ER.
中文翻译:

ER 酶 FICD 中的寡聚状态依赖性开关调节 BiP 的 AMPylation 和 deAMPylation。
AMPylation 是一种失活修饰,可改变主要内质网 (ER) 伴侣 BiP 的活性,以匹配未折叠蛋白的负担。单一内质网定位的 Fic 蛋白 FICD (HYPE) 可催化 BiP 的 AMPylation 和 deAMPylation。然而,FICD 活动转变的基础尚不清楚。我们报告了 FICD 从使 BiP 去 AMP 化的二聚酶转变为具有有效 AMP 化活性的单体。二聚体界面的突变,或连接二聚体界面与酶活性位点的抑制途径残基的突变,有利于体外和细胞内的 BiP AMPylation。从机制上讲,单体化缓解了从二聚体界面变构传播到抑制性 Glu234 的抑制效应,从而允许 MgATP 进行 AMPylation 结合。此外,从核苷酸结合位点传播的相互信号提供了将 FICD 的寡聚状态和酶活性与 ER 的能量状态耦合的机制。
更新日期:2019-11-06
中文翻译:

ER 酶 FICD 中的寡聚状态依赖性开关调节 BiP 的 AMPylation 和 deAMPylation。
AMPylation 是一种失活修饰,可改变主要内质网 (ER) 伴侣 BiP 的活性,以匹配未折叠蛋白的负担。单一内质网定位的 Fic 蛋白 FICD (HYPE) 可催化 BiP 的 AMPylation 和 deAMPylation。然而,FICD 活动转变的基础尚不清楚。我们报告了 FICD 从使 BiP 去 AMP 化的二聚酶转变为具有有效 AMP 化活性的单体。二聚体界面的突变,或连接二聚体界面与酶活性位点的抑制途径残基的突变,有利于体外和细胞内的 BiP AMPylation。从机制上讲,单体化缓解了从二聚体界面变构传播到抑制性 Glu234 的抑制效应,从而允许 MgATP 进行 AMPylation 结合。此外,从核苷酸结合位点传播的相互信号提供了将 FICD 的寡聚状态和酶活性与 ER 的能量状态耦合的机制。















































 京公网安备 11010802027423号
京公网安备 11010802027423号