当前位置:
X-MOL 学术
›
ACS Macro Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Synthesis of Rapidly Surface Eroding Polyorthoesters and Polyacetals Using Thiol–ene Click Chemistry
ACS Macro Letters ( IF 5.1 ) Pub Date : 2019-09-17 , DOI: 10.1021/acsmacrolett.9b00463 Gordon Herwig 1, 2 , Andrew P Dove 1
ACS Macro Letters ( IF 5.1 ) Pub Date : 2019-09-17 , DOI: 10.1021/acsmacrolett.9b00463 Gordon Herwig 1, 2 , Andrew P Dove 1
Affiliation
|
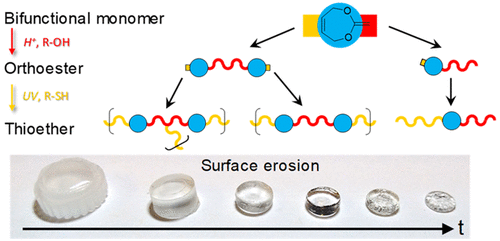
Polyorthoesters are generally considered to be highly biocompatible, surface-eroding materials. However, sensitive intermediates and poor mechanical performance have largely prevented their widespread application to date. Herein, a simple and versatile method to synthesize orthoester- and acetal-based polymers is presented. Using 2-methylene-1,3-dioxe-5-pene as a stable bifunctional monomer, sequential highly selective “click” reactions led initially to the formation of orthoesters (OE) in a Markovnikov alcohol addition or acetals via anti-Markovnikov thiol–ene addition. Subsequent photoinitiated thiol addition onto the remaining endocyclic and backbone alkene functionalities lead to thioether formation to produce a class of poly(orthoester-thioether)s or poly(acetal-thioether)s via a step-growth polymerization. While all obtained polymers were found to possess a weight-average molecular weight of above 10 kg·mol–1, the application of an OE monomer with additional double bond functionality led to a cross-linked polymer network which displayed surface-erosion behavior.
中文翻译:

使用硫醇-烯点击化学合成快速表面侵蚀的聚原酸酯和聚缩醛
聚原酸酯通常被认为是高度生物相容的表面侵蚀材料。然而,敏感的中间体和较差的机械性能在很大程度上阻碍了它们迄今为止的广泛应用。本文介绍了一种简单而通用的合成原酸酯基和缩醛基聚合物的方法。使用 2-methylene-1,3-dioxe-5-pene 作为稳定的双功能单体,连续的高选择性“点击”反应最初导致在 Markovnikov 醇加成中形成原酸酯 (OE) 或通过抗 Markovnikov 硫醇形成缩醛–烯加成。随后将光引发的硫醇加成到剩余的环内和骨架烯烃官能团上,导致硫醚形成,通过逐步聚合产生一类聚(原酸酯-硫醚)或聚(缩醛-硫醚)。–1,具有额外双键功能的 OE 单体的应用导致交联聚合物网络显示出表面侵蚀行为。
更新日期:2019-09-18
中文翻译:

使用硫醇-烯点击化学合成快速表面侵蚀的聚原酸酯和聚缩醛
聚原酸酯通常被认为是高度生物相容的表面侵蚀材料。然而,敏感的中间体和较差的机械性能在很大程度上阻碍了它们迄今为止的广泛应用。本文介绍了一种简单而通用的合成原酸酯基和缩醛基聚合物的方法。使用 2-methylene-1,3-dioxe-5-pene 作为稳定的双功能单体,连续的高选择性“点击”反应最初导致在 Markovnikov 醇加成中形成原酸酯 (OE) 或通过抗 Markovnikov 硫醇形成缩醛–烯加成。随后将光引发的硫醇加成到剩余的环内和骨架烯烃官能团上,导致硫醚形成,通过逐步聚合产生一类聚(原酸酯-硫醚)或聚(缩醛-硫醚)。–1,具有额外双键功能的 OE 单体的应用导致交联聚合物网络显示出表面侵蚀行为。






































 京公网安备 11010802027423号
京公网安备 11010802027423号