当前位置:
X-MOL 学术
›
ACS Appl. Energy Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Redox-State-Dependent Interplay between Pendant Group and Conducting Polymer Backbone in Quinone-Based Conducting Redox Polymers for Lithium Ion Batteries
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2019-09-16 00:00:00 , DOI: 10.1021/acsaem.9b01130 Huan Wang 1 , Rikard Emanuelsson 1 , Haidong Liu 2 , Kristina Edström 2 , Fikret Mamedov 2 , Maria Strømme 1 , Martin Sjödin 1
ACS Applied Energy Materials ( IF 5.4 ) Pub Date : 2019-09-16 00:00:00 , DOI: 10.1021/acsaem.9b01130 Huan Wang 1 , Rikard Emanuelsson 1 , Haidong Liu 2 , Kristina Edström 2 , Fikret Mamedov 2 , Maria Strømme 1 , Martin Sjödin 1
Affiliation
|
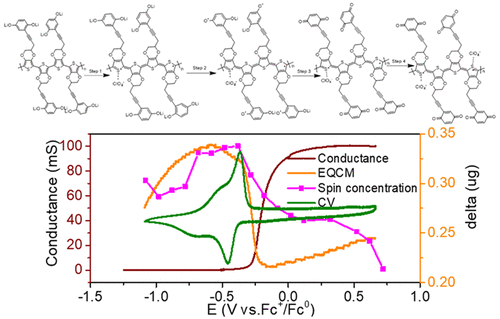
Conducting redox polymers (CRPs) have attracted increased interest in recent years because of the possibility of combining the favorable electron-transport properties of conducting polymers with the additional functionality provided by the redox active pendant groups (PGs). Herein we present a series of quinone-substituted PEDOT-CRPs where the quinone PGs have been substituted by electron-withdrawing substituents. Introducing electron-withdrawing substituents leads to an increase of the quinone formal potential, making, for example, CF3-substituted CRPs, a promising high-voltage cathode material for lithium ion batteries with a well-defined charge/discharge plateau around 3 V vs Li+/Li0. Interestingly, we find a shift in conductance onset potential concomitant with the quinone formal potential shift, indicating that the polymer backbone conductance is intimately associated with the PG redox chemistry. Through in situ UV–vis, electron paramagnetic resonance (EPR), and electrochemical quartz crystal microbalance experiments as well as by experiments in lithium- and tert-butyl-ammonium-based electrolytes, we show that the conductance delay is caused by the reduced lithiated quinone state, most likely by localizing the polaron charge carrier as indicated by EPR and UV–vis experiments.
中文翻译:

锂离子电池基于醌的导电氧化还原聚合物中的侧链基团与导电聚合物骨架之间的氧化还原状态依赖性相互作用
近年来,由于导电聚合物的良好电子传输性能与氧化还原活性侧基(PGs)提供的附加功能相结合的可能性,导电氧化还原聚合物(CRP)引起了越来越多的关注。本文中,我们介绍了一系列的醌取代的PEDOT-CRP,其中醌PG已被吸电子取代基取代。引入吸电子取代基导致醌形式电势的增加,例如,使CF 3取代的CRPs成为用于锂离子电池的有希望的高压阴极材料,具有明确的充放电平台,约为3 V vs.锂+ /锂0。有趣的是,我们发现电导的起始电势随醌形式电势的变化而变化,表明聚合物主链电导与PG氧化还原化学密切相关。通过原位紫外可见光,电子顺磁共振(EPR)和电化学石英晶体微天平实验以及在锂和叔丁基铵基电解质中的实验,我们表明电导延迟是由减少的锂化引起的。醌状态,很可能是通过定位极化子载流子来实现的,如EPR和UV-vis实验所示。
更新日期:2019-09-16
中文翻译:

锂离子电池基于醌的导电氧化还原聚合物中的侧链基团与导电聚合物骨架之间的氧化还原状态依赖性相互作用
近年来,由于导电聚合物的良好电子传输性能与氧化还原活性侧基(PGs)提供的附加功能相结合的可能性,导电氧化还原聚合物(CRP)引起了越来越多的关注。本文中,我们介绍了一系列的醌取代的PEDOT-CRP,其中醌PG已被吸电子取代基取代。引入吸电子取代基导致醌形式电势的增加,例如,使CF 3取代的CRPs成为用于锂离子电池的有希望的高压阴极材料,具有明确的充放电平台,约为3 V vs.锂+ /锂0。有趣的是,我们发现电导的起始电势随醌形式电势的变化而变化,表明聚合物主链电导与PG氧化还原化学密切相关。通过原位紫外可见光,电子顺磁共振(EPR)和电化学石英晶体微天平实验以及在锂和叔丁基铵基电解质中的实验,我们表明电导延迟是由减少的锂化引起的。醌状态,很可能是通过定位极化子载流子来实现的,如EPR和UV-vis实验所示。






























 京公网安备 11010802027423号
京公网安备 11010802027423号