当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thioarsenite Detection and Implications for Arsenic Transport in Groundwater
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2019-09-26 , DOI: 10.1021/acs.est.9b04478 Richard T Wilkin 1 , Robert G Ford 2 , Lisa M Costantino 1 , Randall R Ross 1 , Douglas G Beak 1 , Kirk G Scheckel 2
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2019-09-26 , DOI: 10.1021/acs.est.9b04478 Richard T Wilkin 1 , Robert G Ford 2 , Lisa M Costantino 1 , Randall R Ross 1 , Douglas G Beak 1 , Kirk G Scheckel 2
Affiliation
|
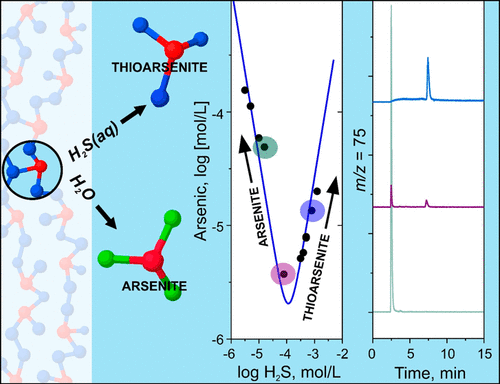
Arsenic toxicity and mobility in groundwater depend on its aqueous speciation. Uncertainty about the methods used for measuring arsenic speciation in sulfate-reducing environments hampers transport and fate analyses and the development of in situ remediation approaches for treating impacted aquifers. New anion-exchange chromatography methods linked to inductively coupled plasma mass spectrometry (ICP-MS) are presented that allow for sample/eluent pH matching. Sample/eluent pH matching is advantageous to prevent thioarsenic species transformation during chromatographic separation because species protonation states remain unaffected, hydroxyl-for-bisulfide ligand substitution is avoided, and oxidation of reduced arsenic species is minimized. We characterized model and natural solutions containing mixtures of arsenic oxyanions with dissolved sulfide and solutions derived from the dissolution of thioarsenite and thioarsenate solids. In sulfidic solutions containing arsenite, two thioarsenic species with S/As ratios of 2:1 and 3:1 were important over the pH range from 5.5 to 8.5. The 3:1 thioarsenic species dominated when disordered As2S3 dissolved into sulfide-containing solution at pH 5.4. Together with the preferential formation of arsenite following sample dilution, these data provide evidence for the formation and detection of thioarsenite species. This study helps resolve inconsistencies between spectroscopic and chromatographic evidence regarding the nature of arsenic in sulfidic waters.
中文翻译:

硫代亚砷酸盐的检测及其对地下水中砷迁移的影响
地下水中砷的毒性和迁移性取决于其水相形态。用于测量硫酸盐还原环境中砷形态的方法的不确定性阻碍了迁移和归宿分析以及处理受影响含水层的原位修复方法的开发。提出了与电感耦合等离子体质谱 (ICP-MS) 相关的新型阴离子交换色谱方法,可实现样品/洗脱液 pH 值匹配。样品/洗脱液 pH 匹配有利于防止色谱分离过程中硫代砷物质的转化,因为物质质子化状态不受影响,避免了羟基对二硫化物配体的取代,并且还原砷物质的氧化被最小化。我们对含有砷氧阴离子与溶解的硫化物的混合物的模型溶液和天然溶液以及由硫代亚砷酸盐和硫代砷酸盐固体溶解衍生的溶液进行了表征。在含有亚砷酸盐的硫化物溶液中,S/As 比例为 2:1 和 3:1 的两种硫代砷物质在 pH 值 5.5 至 8.5 范围内非常重要。当无序As 2 S 3溶解到pH 5.4的含硫化物溶液中时,3:1硫代砷形态占主导地位。连同样品稀释后亚砷酸盐的优先形成,这些数据为硫代亚砷酸盐物质的形成和检测提供了证据。这项研究有助于解决有关硫化物水中砷性质的光谱和色谱证据之间的不一致问题。
更新日期:2019-09-26
中文翻译:

硫代亚砷酸盐的检测及其对地下水中砷迁移的影响
地下水中砷的毒性和迁移性取决于其水相形态。用于测量硫酸盐还原环境中砷形态的方法的不确定性阻碍了迁移和归宿分析以及处理受影响含水层的原位修复方法的开发。提出了与电感耦合等离子体质谱 (ICP-MS) 相关的新型阴离子交换色谱方法,可实现样品/洗脱液 pH 值匹配。样品/洗脱液 pH 匹配有利于防止色谱分离过程中硫代砷物质的转化,因为物质质子化状态不受影响,避免了羟基对二硫化物配体的取代,并且还原砷物质的氧化被最小化。我们对含有砷氧阴离子与溶解的硫化物的混合物的模型溶液和天然溶液以及由硫代亚砷酸盐和硫代砷酸盐固体溶解衍生的溶液进行了表征。在含有亚砷酸盐的硫化物溶液中,S/As 比例为 2:1 和 3:1 的两种硫代砷物质在 pH 值 5.5 至 8.5 范围内非常重要。当无序As 2 S 3溶解到pH 5.4的含硫化物溶液中时,3:1硫代砷形态占主导地位。连同样品稀释后亚砷酸盐的优先形成,这些数据为硫代亚砷酸盐物质的形成和检测提供了证据。这项研究有助于解决有关硫化物水中砷性质的光谱和色谱证据之间的不一致问题。

































 京公网安备 11010802027423号
京公网安备 11010802027423号