Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
SLC1A3 contributes to L-asparaginase resistance in solid tumors.
The EMBO Journal ( IF 9.4 ) Pub Date : 2019-09-16 , DOI: 10.15252/embj.2019102147 Jianhui Sun 1, 2 , Remco Nagel 1 , Esther A Zaal 3 , Alejandro Piñeiro Ugalde 1 , Ruiqi Han 1, 2 , Natalie Proost 4 , Ji-Ying Song 5 , Abhijeet Pataskar 1 , Artur Burylo 6 , Haigen Fu 7 , Gerrit J Poelarends 7 , Marieke van de Ven 4 , Olaf van Tellingen 6 , Celia R Berkers 3, 8 , Reuven Agami 1, 2
The EMBO Journal ( IF 9.4 ) Pub Date : 2019-09-16 , DOI: 10.15252/embj.2019102147 Jianhui Sun 1, 2 , Remco Nagel 1 , Esther A Zaal 3 , Alejandro Piñeiro Ugalde 1 , Ruiqi Han 1, 2 , Natalie Proost 4 , Ji-Ying Song 5 , Abhijeet Pataskar 1 , Artur Burylo 6 , Haigen Fu 7 , Gerrit J Poelarends 7 , Marieke van de Ven 4 , Olaf van Tellingen 6 , Celia R Berkers 3, 8 , Reuven Agami 1, 2
Affiliation
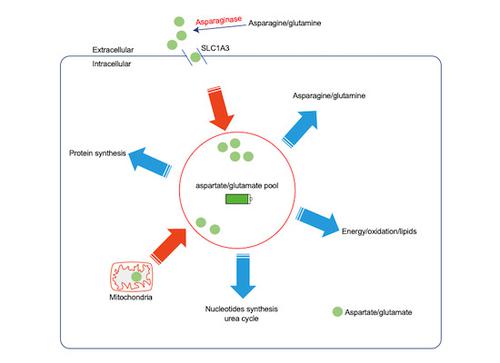
|
L-asparaginase (ASNase) serves as an effective drug for adolescent acute lymphoblastic leukemia. However, many clinical trials indicated severe ASNase toxicity in patients with solid tumors, with resistant mechanisms not well understood. Here, we took a functional genetic approach and identified SLC1A3 as a novel contributor to ASNase resistance in cancer cells. In combination with ASNase, SLC1A3 inhibition caused cell cycle arrest or apoptosis, and myriads of metabolic vulnerabilities in tricarboxylic acid (TCA) cycle, urea cycle, nucleotides biosynthesis, energy production, redox homeostasis, and lipid biosynthesis. SLC1A3 is an aspartate and glutamate transporter, mainly expressed in brain tissues, but high expression levels were also observed in some tumor types. Here, we demonstrate that ASNase stimulates aspartate and glutamate consumptions, and their refilling through SLC1A3 promotes cancer cell proliferation. Lastly, in vivo experiments indicated that SLC1A3 expression promoted tumor development and metastasis while negating the suppressive effects of ASNase by fueling aspartate, glutamate, and glutamine metabolisms despite of asparagine shortage. Altogether, our findings identify a novel role for SLC1A3 in ASNase resistance and suggest that restrictive aspartate and glutamate uptake might improve ASNase efficacy with solid tumors.
中文翻译:

SLC1A3 有助于实体瘤中的 L-天冬酰胺酶抵抗。
L-天冬酰胺酶(ASNase)是治疗青少年急性淋巴细胞白血病的有效药物。然而,许多临床试验表明实体瘤患者存在严重的 ASNase 毒性,且耐药机制尚不清楚。在这里,我们采用功能遗传学方法,并将 SLC1A3 确定为癌细胞中 ASNase 抗性的新贡献者。与 ASNase 结合,SLC1A3 抑制导致细胞周期停滞或凋亡,以及三羧酸 (TCA) 循环、尿素循环、核苷酸生物合成、能量产生、氧化还原稳态和脂质生物合成中的无数代谢脆弱性。 SLC1A3是一种天冬氨酸和谷氨酸转运蛋白,主要在脑组织中表达,但在某些肿瘤类型中也观察到高表达水平。在这里,我们证明 ASNase 会刺激天冬氨酸和谷氨酸的消耗,而它们通过 SLC1A3 的补充会促进癌细胞增殖。最后,体内实验表明,尽管天冬酰胺短缺,但 SLC1A3 表达促进了肿瘤的发展和转移,同时通过促进天冬氨酸、谷氨酸和谷氨酰胺代谢而抵消了 ASNase 的抑制作用。总而言之,我们的研究结果确定了 SLC1A3 在 ASNase 耐药性中的新作用,并表明限制天冬氨酸和谷氨酸的摄取可能会提高 ASNase 对实体瘤的疗效。
更新日期:2019-11-06
中文翻译:

SLC1A3 有助于实体瘤中的 L-天冬酰胺酶抵抗。
L-天冬酰胺酶(ASNase)是治疗青少年急性淋巴细胞白血病的有效药物。然而,许多临床试验表明实体瘤患者存在严重的 ASNase 毒性,且耐药机制尚不清楚。在这里,我们采用功能遗传学方法,并将 SLC1A3 确定为癌细胞中 ASNase 抗性的新贡献者。与 ASNase 结合,SLC1A3 抑制导致细胞周期停滞或凋亡,以及三羧酸 (TCA) 循环、尿素循环、核苷酸生物合成、能量产生、氧化还原稳态和脂质生物合成中的无数代谢脆弱性。 SLC1A3是一种天冬氨酸和谷氨酸转运蛋白,主要在脑组织中表达,但在某些肿瘤类型中也观察到高表达水平。在这里,我们证明 ASNase 会刺激天冬氨酸和谷氨酸的消耗,而它们通过 SLC1A3 的补充会促进癌细胞增殖。最后,体内实验表明,尽管天冬酰胺短缺,但 SLC1A3 表达促进了肿瘤的发展和转移,同时通过促进天冬氨酸、谷氨酸和谷氨酰胺代谢而抵消了 ASNase 的抑制作用。总而言之,我们的研究结果确定了 SLC1A3 在 ASNase 耐药性中的新作用,并表明限制天冬氨酸和谷氨酸的摄取可能会提高 ASNase 对实体瘤的疗效。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号