当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Thermodynamic Assessment of Coating Materials for Solid-State Li, Na, and K Batteries
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-09-30 , DOI: 10.1021/acsami.9b11001 Seungho Yu 1 , Haesun Park , Donald J. Siegel
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2019-09-30 , DOI: 10.1021/acsami.9b11001 Seungho Yu 1 , Haesun Park , Donald J. Siegel
Affiliation
|
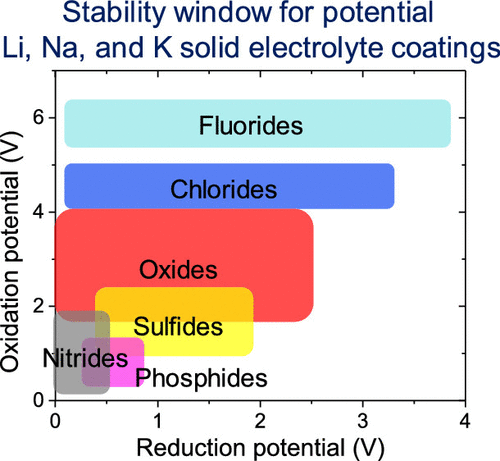
The development of all-solid-state batteries (ASSBs) presents a pathway to enhance the energy density and safety of conventional Li-ion batteries that use liquid electrolytes. However, one of the more promising categories of solid electrolytes (SEs), sulfides, are generally unstable in contact with common electrode materials, resulting in SE decomposition and high interfacial resistance. Recent studies have indicated that the application of coatings can, in some cases, stabilize the electrode/SE interface, reducing the likelihood for harmful interfacial reactions. Here, stable coatings for Li, Na, and K ASSBs are identified. In total, the stability windows for 1112 ternary alkali-metal-based compounds were assessed, including fluorides, chlorides, oxides, sulfides, phosphides, and nitrides. In general, the fluorides and chlorides exhibit the highest oxidative stability, suggesting that they are good choices for stabilizing SE/cathode interfaces. In contrast, sulfides, phosphides, and nitrides exhibit much lower oxidative stabilities, with many of these materials predicted to decompose above 2 V. At the anode/SE interface, nitrides and oxides are predicted to be the most effective coatings, as they are generally the most stable against reductive decomposition. As expected, sulfides and phosphides are the least stable class of materials under reducing conditions. Overall, oxides appear to be the most versatile class of coating materials: several oxides are predicted to exhibit stability windows ranging from 0 to 3 V with respect to Li/Li+, Na/Na+, or K/K+. Examples of promising oxides for stabilizing the SE/anode interface include Li5AlO4, Li4SiO4, NaAlO2, Na3PO4, KAlO2, and K3PO4. Similarly, promising compounds for stabilizing the SE/cathode interface include NaPO3 and KPO3. Finally, the possibility for kinetic stabilization suggests that additional ternary oxides (e.g., based on Ga, Nb, Sb, and Ta) may be viable coatings at the SE/cathode interface.
中文翻译:

固态锂,钠和钾电池涂层材料的热力学评估
全固态电池(ASSB)的发展为提高使用液体电解质的传统锂离子电池的能量密度和安全性提供了一条途径。但是,固态电解质(SEs)最具前景的一种类别是硫化物,通常在与普通电极材料接触时不稳定,从而导致SE分解和高界面电阻。最近的研究表明,在某些情况下,涂层的使用可以稳定电极/ SE界面,减少有害的界面反应的可能性。在此,确定了用于Li,Na和K ASSB的稳定涂层。总共评估了1112种三元碱金属化合物的稳定性窗口,包括氟化物,氯化物,氧化物,硫化物,磷化物和氮化物。一般来说,氟化物和氯化物具有最高的氧化稳定性,表明它们是稳定SE /阴极界面的良好选择。相比之下,硫化物,磷化物和氮化物的氧化稳定性要低得多,其中许多材料预计会在2 V以上分解。在阳极/ SE界面,氮化物和氧化物被认为是最有效的涂层,因为它们通常是对还原分解最稳定。不出所料,在还原条件下,硫化物和磷化物是最不稳定的材料类别。总体而言,氧化物似乎是涂料中用途最广泛的一类:相对于Li / Li,预计几种氧化物的稳定性窗口范围为0至3 V 硫化物,磷化物和氮化物的氧化稳定性要低得多,其中许多材料预计会在2 V以上分解。在阳极/ SE界面,氮化物和氧化物被认为是最有效的涂层,因为它们通常是最稳定的反对还原分解。不出所料,在还原条件下,硫化物和磷化物是最不稳定的材料类别。总体而言,氧化物似乎是涂料中用途最广泛的一类:相对于Li / Li,预计几种氧化物的稳定性窗口范围为0至3 V 硫化物,磷化物和氮化物的氧化稳定性要低得多,其中许多材料预计会在2 V以上分解。在阳极/ SE界面,氮化物和氧化物被认为是最有效的涂层,因为它们通常是最稳定的反对还原分解。不出所料,在还原条件下,硫化物和磷化物是最不稳定的材料类别。总体而言,氧化物似乎是涂料中用途最广泛的一类:相对于Li / Li,预计几种氧化物的稳定性窗口范围为0至3 V 因为它们通常对还原分解最稳定。不出所料,在还原条件下,硫化物和磷化物是最不稳定的材料类别。总体而言,氧化物似乎是涂料中用途最广泛的一类:相对于Li / Li,预计几种氧化物的稳定性窗口范围为0至3 V 因为它们通常对还原分解最稳定。不出所料,在还原条件下,硫化物和磷化物是最不稳定的材料类别。总体而言,氧化物似乎是涂料中用途最广泛的一类:相对于Li / Li,预计几种氧化物的稳定性窗口范围为0至3 V+,Na / Na +或K / K +。用于稳定SE /阳极界面的有希望的氧化物的实例包括Li 5 AlO 4,Li 4 SiO 4,NaAlO 2,Na 3 PO 4,KAlO 2和K 3 PO 4。类似地,用于稳定SE /阴极界面的有前途的化合物包括NaPO 3和KPO 3。最后,动力学稳定的可能性表明,其他三元氧化物(例如基于Ga,Nb,Sb和Ta的氧化物)在SE /阴极界面处可能是可行的涂层。
更新日期:2019-10-01
中文翻译:

固态锂,钠和钾电池涂层材料的热力学评估
全固态电池(ASSB)的发展为提高使用液体电解质的传统锂离子电池的能量密度和安全性提供了一条途径。但是,固态电解质(SEs)最具前景的一种类别是硫化物,通常在与普通电极材料接触时不稳定,从而导致SE分解和高界面电阻。最近的研究表明,在某些情况下,涂层的使用可以稳定电极/ SE界面,减少有害的界面反应的可能性。在此,确定了用于Li,Na和K ASSB的稳定涂层。总共评估了1112种三元碱金属化合物的稳定性窗口,包括氟化物,氯化物,氧化物,硫化物,磷化物和氮化物。一般来说,氟化物和氯化物具有最高的氧化稳定性,表明它们是稳定SE /阴极界面的良好选择。相比之下,硫化物,磷化物和氮化物的氧化稳定性要低得多,其中许多材料预计会在2 V以上分解。在阳极/ SE界面,氮化物和氧化物被认为是最有效的涂层,因为它们通常是对还原分解最稳定。不出所料,在还原条件下,硫化物和磷化物是最不稳定的材料类别。总体而言,氧化物似乎是涂料中用途最广泛的一类:相对于Li / Li,预计几种氧化物的稳定性窗口范围为0至3 V 硫化物,磷化物和氮化物的氧化稳定性要低得多,其中许多材料预计会在2 V以上分解。在阳极/ SE界面,氮化物和氧化物被认为是最有效的涂层,因为它们通常是最稳定的反对还原分解。不出所料,在还原条件下,硫化物和磷化物是最不稳定的材料类别。总体而言,氧化物似乎是涂料中用途最广泛的一类:相对于Li / Li,预计几种氧化物的稳定性窗口范围为0至3 V 硫化物,磷化物和氮化物的氧化稳定性要低得多,其中许多材料预计会在2 V以上分解。在阳极/ SE界面,氮化物和氧化物被认为是最有效的涂层,因为它们通常是最稳定的反对还原分解。不出所料,在还原条件下,硫化物和磷化物是最不稳定的材料类别。总体而言,氧化物似乎是涂料中用途最广泛的一类:相对于Li / Li,预计几种氧化物的稳定性窗口范围为0至3 V 因为它们通常对还原分解最稳定。不出所料,在还原条件下,硫化物和磷化物是最不稳定的材料类别。总体而言,氧化物似乎是涂料中用途最广泛的一类:相对于Li / Li,预计几种氧化物的稳定性窗口范围为0至3 V 因为它们通常对还原分解最稳定。不出所料,在还原条件下,硫化物和磷化物是最不稳定的材料类别。总体而言,氧化物似乎是涂料中用途最广泛的一类:相对于Li / Li,预计几种氧化物的稳定性窗口范围为0至3 V+,Na / Na +或K / K +。用于稳定SE /阳极界面的有希望的氧化物的实例包括Li 5 AlO 4,Li 4 SiO 4,NaAlO 2,Na 3 PO 4,KAlO 2和K 3 PO 4。类似地,用于稳定SE /阴极界面的有前途的化合物包括NaPO 3和KPO 3。最后,动力学稳定的可能性表明,其他三元氧化物(例如基于Ga,Nb,Sb和Ta的氧化物)在SE /阴极界面处可能是可行的涂层。






































 京公网安备 11010802027423号
京公网安备 11010802027423号