当前位置:
X-MOL 学术
›
Mol. Pharmaceutics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
111In- and 225Ac-Labeled Cixutumumab for Imaging and α-Particle Radiotherapy of IGF-1R Positive Triple-Negative Breast Cancer.
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2019-11-05 , DOI: 10.1021/acs.molpharmaceut.9b00542 Viswas Raja Solomon , Elahe Alizadeh , Wendy Bernhard , Siddesh V Hartimath , Wayne Hill , Rufael Chekol , Kris M Barreto , Clarence Ronald Geyer , Humphrey Fonge 1
Molecular Pharmaceutics ( IF 4.5 ) Pub Date : 2019-11-05 , DOI: 10.1021/acs.molpharmaceut.9b00542 Viswas Raja Solomon , Elahe Alizadeh , Wendy Bernhard , Siddesh V Hartimath , Wayne Hill , Rufael Chekol , Kris M Barreto , Clarence Ronald Geyer , Humphrey Fonge 1
Affiliation
|
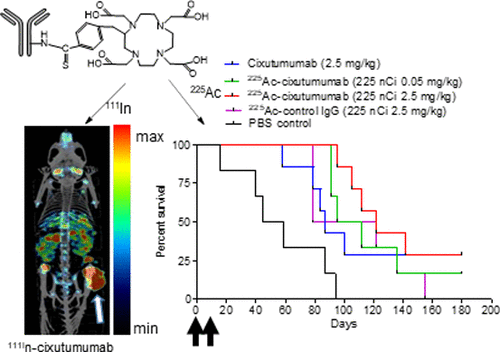
Insulin growth factor receptor (IGF-1R) is overexpressed in many cancers of epithelial origin, where it confers enhanced proliferation and resistance to therapies targeted at other receptors. Anti-IGF-1R monoclonal antibodies have not demonstrated significant improvements in patient outcomes in clinical trials. Humanized monoclonal antibody cixutumumab (IMC-A12) binds to IGF-1R with low nM affinity. In this study, cixutumumab was conjugated with p-SCN-Bn-DOTA and radiolabeled with 111In or 225Ac for imaging or radiotherapy using a triple-negative breast cancer (TNBC) model SUM149PT. The antibody conjugate showed low nM affinity to IGF-1R, which was not affected by conjugation and radiolabeling procedures. Cixutumumab immunoconjugates were effectively internalized in SUM149PT and were cytotoxic to the cells with an EC50 of 225Ac–cixutumumab (0.02 nM) that was almost 5000-fold less than that of unlabeled cixutumumab (95.2 nM). MicroSPECT imaging of the SUM149PT xenograft showed the highest tumor uptake occurred at 48 h post injection and was 9.9 ± 0.5% injected activity per gram (%IA/cc). In radiotherapy studies, we evaluated the effect of the specific activity of 225Ac–cixutumumab on efficacy following a tail vein injection of two doses (days 0 and 10) of the investigation agent or controls. Cixutumumab (2.5 mg/kg) prolonged the survival of the SUM149PT tumor-bearing mice with a median survival of 87 days compared to the PBS control group (median survival of 62 days). Median survival of high specific activity 225Ac–cixutumumab (8 kBq/μg, 225 nCi, 0.05 mg/kg) was 103.5 days compared to 122 days for low specific activity 225Ac–cixutumumab (0.15 kBq/μg, 225 nCi, 2.5 mg/kg). Additionally, low specific activity radioimmunoconjugate led to complete tumor remission in 2/6 mice. The data suggest that the efficacy of cixutumumab can be enhanced by radiolabeling with 225Ac at a low specific activity.
中文翻译:

111In和225Ac标记的Cixutumumab用于IGF-1R阳性三阴性乳腺癌的成像和α粒子放疗。
胰岛素生长因子受体(IGF-1R)在许多上皮起源的癌症中都过表达,在这种情况下,它赋予增强的增殖能力和对针对其他受体的疗法的抵抗力。在临床试验中,抗IGF-1R单克隆抗体并未显示出患者预后的显着改善。人源化单克隆抗体cixutumumab(IMC-A12)以低nM亲和力与IGF-1R结合。在这项研究中,西妥珠单抗与p -SCN-Bn-DOTA偶联,并用111 In或225进行放射性标记使用三阴性乳腺癌(TNBC)模型SUM149PT进行成像或放射治疗的Ac。抗体结合物显示出对IGF-1R的低nM亲和力,不受结合和放射标记程序的影响。西妥昔单抗免疫偶联物在SUM149PT中有效内在化,对细胞具有细胞毒性,EC 50为225 Ac–西妥昔单抗(0.02 nM),几乎比未标记西妥昔单抗(95.2 nM)低5000倍。SUM149PT异种移植物的MicroSPECT成像显示,最高的肿瘤吸收发生在注射后48小时,注射活性为9.9±0.5%/克(%IA / cc)。在放疗研究中,我们评估了225的比活的影响Ac-cixutumumab对尾静脉注射两种剂量(第0天和第10天)的研究药物或对照组的疗效。与PBS对照组相比,西妥珠单抗(2.5 mg / kg)延长了SUM149PT荷瘤小鼠的生存期,中位生存期为87天(中位生存期为62天)。高比活度225 Ac–cixutumumab(8 kBq /μg,225 nCi,0.05 mg / kg)的中位生存期为103.5天,而低比活度225 Ac–cixutumumab(0.15 kBq /μg,225 nCi,2.5 mg )的中位生存期为103.5天/公斤)。此外,低比活度放射免疫缀合物导致2/6小鼠的肿瘤完全缓解。数据表明,通过以低比活度用225 Ac进行放射性标记可以增强西妥昔单抗的功效。
更新日期:2019-11-05
中文翻译:

111In和225Ac标记的Cixutumumab用于IGF-1R阳性三阴性乳腺癌的成像和α粒子放疗。
胰岛素生长因子受体(IGF-1R)在许多上皮起源的癌症中都过表达,在这种情况下,它赋予增强的增殖能力和对针对其他受体的疗法的抵抗力。在临床试验中,抗IGF-1R单克隆抗体并未显示出患者预后的显着改善。人源化单克隆抗体cixutumumab(IMC-A12)以低nM亲和力与IGF-1R结合。在这项研究中,西妥珠单抗与p -SCN-Bn-DOTA偶联,并用111 In或225进行放射性标记使用三阴性乳腺癌(TNBC)模型SUM149PT进行成像或放射治疗的Ac。抗体结合物显示出对IGF-1R的低nM亲和力,不受结合和放射标记程序的影响。西妥昔单抗免疫偶联物在SUM149PT中有效内在化,对细胞具有细胞毒性,EC 50为225 Ac–西妥昔单抗(0.02 nM),几乎比未标记西妥昔单抗(95.2 nM)低5000倍。SUM149PT异种移植物的MicroSPECT成像显示,最高的肿瘤吸收发生在注射后48小时,注射活性为9.9±0.5%/克(%IA / cc)。在放疗研究中,我们评估了225的比活的影响Ac-cixutumumab对尾静脉注射两种剂量(第0天和第10天)的研究药物或对照组的疗效。与PBS对照组相比,西妥珠单抗(2.5 mg / kg)延长了SUM149PT荷瘤小鼠的生存期,中位生存期为87天(中位生存期为62天)。高比活度225 Ac–cixutumumab(8 kBq /μg,225 nCi,0.05 mg / kg)的中位生存期为103.5天,而低比活度225 Ac–cixutumumab(0.15 kBq /μg,225 nCi,2.5 mg )的中位生存期为103.5天/公斤)。此外,低比活度放射免疫缀合物导致2/6小鼠的肿瘤完全缓解。数据表明,通过以低比活度用225 Ac进行放射性标记可以增强西妥昔单抗的功效。































 京公网安备 11010802027423号
京公网安备 11010802027423号