Nature Communications ( IF 14.7 ) Pub Date : 2019-09-13 , DOI: 10.1038/s41467-019-12181-x Zhong-Jian Cai 1 , Chen-Xu Liu 1 , Qiang Wang 1 , Qing Gu 1 , Shu-Li You 1
|
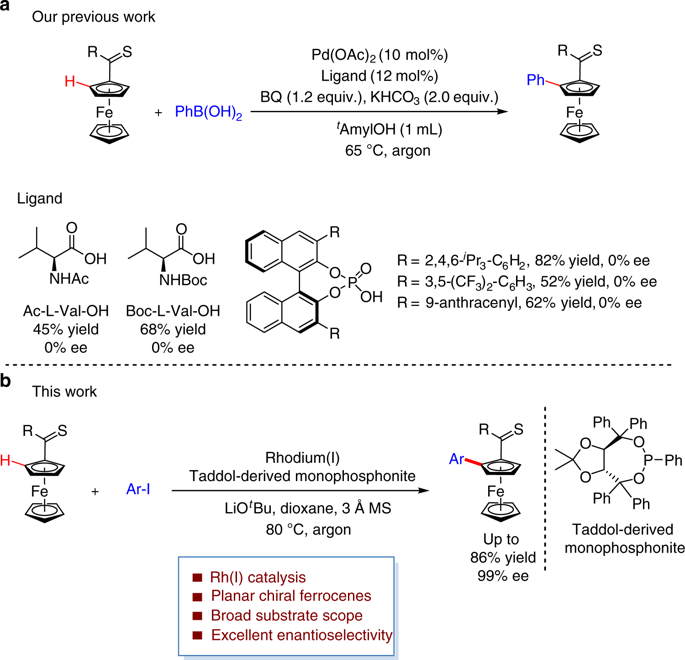
Planar chiral ferrocenes have received great attention in both academia and industry. Although remarkable progresses have been made over the past decade, the development of efficient and straightforward methods for the synthesis of enantiopure planar chiral ferrocenes remains highly challenging. Herein, we report a rhodium(I)/phosphonite catalyzed thioketone-directed enantioselective C-H bond arylation of ferrocenes. Readily available aryl iodides are used as the coupling partners in this transformation, leading to a series of planar chiral ferrocenes in good yields and excellent enantioselectivities (up to 86% yield, 99% ee). Of particular note, heteroaryl coupled ferrocenes, which are difficult to access with previous approaches, can be obtained in satisfactory results.
中文翻译:

噻酮指导的铑(I)催化二茂铁的对映选择性CH键芳基化。
平面手性二茂铁在学术界和工业界都受到了极大的关注。尽管在过去的十年中已经取得了显着的进展,但是开发合成对映纯的平面手性二茂铁的有效而直接的方法仍然具有很高的挑战性。在本文中,我们报道了二茂铁的铑(I)/亚膦酸酯催化的硫代酮定向的对映选择性CH键芳基化。在此转化过程中,现成的芳基碘化物被用作偶联伙伴,从而以高收率和出色的对映选择性(高达86%的收率,99%ee的收率)产生了一系列平面手性二茂铁。特别要注意的是,杂芳基偶联的二茂铁可以用令人满意的结果获得,这些杂芳基偶联的二茂铁很难用以前的方法获得。































 京公网安备 11010802027423号
京公网安备 11010802027423号