当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Liquid-Phase Oxidation of Ethylene Glycol on Pt and Pt–Fe Catalysts for the Production of Glycolic Acid: Remarkable Bimetallic Effect and Reaction Mechanism
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-09-25 , DOI: 10.1021/acs.iecr.9b03419
Honghong Shi 1 , Xiaogang Yin 2 , Bala Subramaniam 1 , Raghunath V. Chaudhari 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2019-09-25 , DOI: 10.1021/acs.iecr.9b03419
Honghong Shi 1 , Xiaogang Yin 2 , Bala Subramaniam 1 , Raghunath V. Chaudhari 1
Affiliation
|
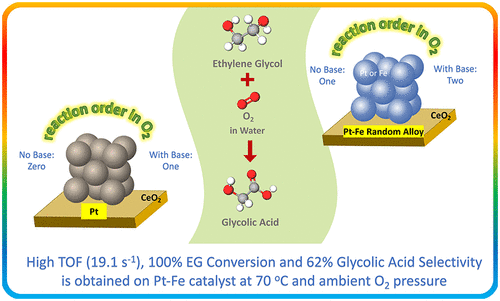
A highly active and selective Pt–Fe alloy catalyst on CeO2 support is reported in this work for aqueous phase oxidation of ethylene glycol (EG) to glycolic acid. The Pt–Fe nanoparticles are highly alloyed with a face-centered cubic (fcc) type of crystal structure and a chemical state of Pt0/Fe0, as confirmed from X-ray diffraction and extended X-ray absorption fine structure characterizations, respectively. Compared to the monometallic Pt catalyst, the Pt–Fe catalyst shows more than a 17-fold higher initial TOF, while achieving complete EG conversion in 4 h at 70 °C and ambient O2 pressure under alkaline conditions. The synergistic bimetallic effect occurs due to significantly changing the O2 adsorption-dissociation characteristics on the catalyst surface. The addition of a base shows a promotional effect on both Pt and Pt–Fe catalysts at low NaOH concentrations but an inhibition effect is observed for both catalysts at sufficiently high NaOH concentrations. Furthermore, the base enhances the synergistic effect observed with Pt–Fe catalyst.
中文翻译:

Pt和Pt-Fe催化剂上的乙二醇液相氧化制备乙醇酸的双金属效应和反应机理
在这项工作中报道了一种在CeO 2载体上的高活性和选择性Pt-Fe合金催化剂,用于将乙二醇(EG)进行水相氧化成乙醇酸。通过X射线衍射和扩展X射线吸收精细结构表征分别证实,Pt-Fe纳米颗粒与面心立方(fcc)型晶体结构和Pt 0 / Fe 0的化学状态高度合金化。与单金属Pt催化剂相比,Pt-Fe催化剂的初始TOF高出17倍以上,同时在70°C和碱性条件下的环境O 2压力下,在4小时内实现了完全EG转化。由于O 2的显着变化,产生了协同双金属效应催化剂表面的吸附-解离特性。碱的添加在低NaOH浓度下对Pt和Pt-Fe催化剂都有促进作用,但在足够高的NaOH浓度下对这两种催化剂均具有抑制作用。此外,碱增强了Pt-Fe催化剂的协同作用。
更新日期:2019-09-26
中文翻译:

Pt和Pt-Fe催化剂上的乙二醇液相氧化制备乙醇酸的双金属效应和反应机理
在这项工作中报道了一种在CeO 2载体上的高活性和选择性Pt-Fe合金催化剂,用于将乙二醇(EG)进行水相氧化成乙醇酸。通过X射线衍射和扩展X射线吸收精细结构表征分别证实,Pt-Fe纳米颗粒与面心立方(fcc)型晶体结构和Pt 0 / Fe 0的化学状态高度合金化。与单金属Pt催化剂相比,Pt-Fe催化剂的初始TOF高出17倍以上,同时在70°C和碱性条件下的环境O 2压力下,在4小时内实现了完全EG转化。由于O 2的显着变化,产生了协同双金属效应催化剂表面的吸附-解离特性。碱的添加在低NaOH浓度下对Pt和Pt-Fe催化剂都有促进作用,但在足够高的NaOH浓度下对这两种催化剂均具有抑制作用。此外,碱增强了Pt-Fe催化剂的协同作用。































 京公网安备 11010802027423号
京公网安备 11010802027423号