当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Inversion of Enantioselectivity in Allene Gas versus Allyl Acetate Reductive Aldehyde Allylation Guided by Metal-Centered Stereogenicity: An Experimental and Computational Study.
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-09-11 , DOI: 10.1021/acscatal.9b03695 Seung Wook Kim 1 , Cole C Meyer 1 , Binh Khanh Mai 2 , Peng Liu 2 , Michael J Krische 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-09-11 , DOI: 10.1021/acscatal.9b03695 Seung Wook Kim 1 , Cole C Meyer 1 , Binh Khanh Mai 2 , Peng Liu 2 , Michael J Krische 1
Affiliation
|
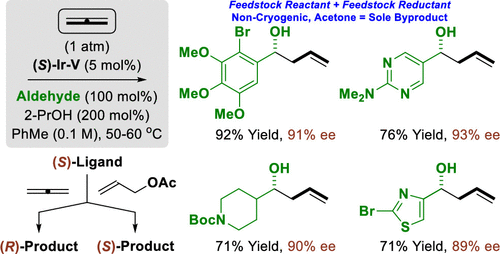
The use of gaseous allene as an allyl pronucleophile in enantioselective aldehyde reductive coupling is described. Notably, using the same antipode of chiral ligand, (S)-tol-BINAP, an inversion of enantioselectivity is observed for allene vs allyl acetate pronucleophiles. Experimental and computational studies corroborate intervention of diastereomeric π-allyliridium-C,O-benzoate complexes, which arise via allene hydrometalation (from a pentacoordinate iridium hydride) vs allyl acetate ionization (from a square planar iridium species).
中文翻译:

在以金属为中心的立体异构性指导下,丙二烯气体中对映选择性与乙酸烯丙酯还原醛的烯丙基化的反演:一项实验和计算研究。
描述了在对映选择性醛还原偶联中使用气态烯丙基作为烯丙基前亲核试剂。值得注意的是,使用相同的手性配体对映体,(S)-tol-BINAP,对于烯丙基乙酸烯丙酯与乙酸烯丙酯亲核试剂观察到对映选择性的反转。实验和计算研究证实了非对映体π-烯丙基-C,O-苯甲酸酯配合物的干预,这是通过烯丙基加氢金属化(来自五配位氢化铱)与乙酸烯丙酯离子化(来自方形平面铱物种)产生的。
更新日期:2019-09-12
中文翻译:

在以金属为中心的立体异构性指导下,丙二烯气体中对映选择性与乙酸烯丙酯还原醛的烯丙基化的反演:一项实验和计算研究。
描述了在对映选择性醛还原偶联中使用气态烯丙基作为烯丙基前亲核试剂。值得注意的是,使用相同的手性配体对映体,(S)-tol-BINAP,对于烯丙基乙酸烯丙酯与乙酸烯丙酯亲核试剂观察到对映选择性的反转。实验和计算研究证实了非对映体π-烯丙基-C,O-苯甲酸酯配合物的干预,这是通过烯丙基加氢金属化(来自五配位氢化铱)与乙酸烯丙酯离子化(来自方形平面铱物种)产生的。































 京公网安备 11010802027423号
京公网安备 11010802027423号