Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cleavage of RIPK1 by caspase-8 is crucial for limiting apoptosis and necroptosis
Nature ( IF 50.5 ) Pub Date : 2019-09-11 , DOI: 10.1038/s41586-019-1548-x
Kim Newton 1 , Katherine E Wickliffe 1 , Debra L Dugger 1 , Allie Maltzman 1 , Merone Roose-Girma 2 , Monika Dohse 3 , László Kőműves 3 , Joshua D Webster 3 , Vishva M Dixit 1
Nature ( IF 50.5 ) Pub Date : 2019-09-11 , DOI: 10.1038/s41586-019-1548-x
Kim Newton 1 , Katherine E Wickliffe 1 , Debra L Dugger 1 , Allie Maltzman 1 , Merone Roose-Girma 2 , Monika Dohse 3 , László Kőműves 3 , Joshua D Webster 3 , Vishva M Dixit 1
Affiliation
|
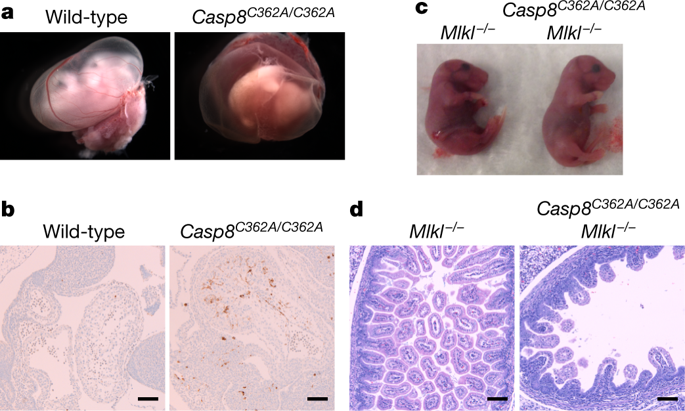
The aspartate-specific cysteine protease caspase-8 suppresses necroptotic cell death mediated by RIPK3 and MLKL. Indeed, mice that lack caspase-8 die in a RIPK3- and MLKL-dependent manner during embryogenesis1–3. In humans, caspase-8 deficiency is associated with immunodeficiency4 or very early onset inflammatory bowel disease5. The substrates that are cleaved by caspase-8 to prevent necroptosis in vivo have not been defined. Here we show that knock-in mice that express catalytically inactive caspase-8(C362A) die as embryos owing to MLKL-dependent necroptosis, similar to caspase-8-deficient mice. Thus, caspase-8 must cleave itself, other proteins or both to inhibit necroptosis. Mice that express caspase-8(D212A/D218A/D225A/D387A), which cannot cleave itself, were viable, as were mice that express c-FLIP or CYLD proteins that had been mutated to prevent cleavage by caspase-8. By contrast, mice that express RIPK1(D325A), in which the caspase-8 cleavage site Asp325 had been mutated, died mid-gestation. Embryonic lethality was prevented by inactivation of RIPK1, loss of TNFR1, or loss of both MLKL and the caspase-8 adaptor FADD, but not by loss of MLKL alone. Thus, RIPK1(D325A) appears to trigger cell death mediated by TNF, the kinase activity of RIPK1 and FADD–caspase-8. Accordingly, dying endothelial cells that contain cleaved caspase-3 were abnormally abundant in yolk sacs of Ripk1D325A/D325A embryos. Heterozygous Ripk1D325A/+ cells and mice were viable, but were also more susceptible to TNF-induced cell death than were wild-type cells or mice. Our data show that Asp325 of RIPK1 is essential for limiting aberrant cell death in response to TNF, consistent with the idea that cleavage of RIPK1 by caspase-8 is a mechanism for dismantling death-inducing complexes.Caspase-8 suppresses apoptosis and necroptosis in embryonic mice by cleaving RIPK1.
中文翻译:

caspase-8 对 RIPK1 的切割对于限制细胞凋亡和坏死性凋亡至关重要
天冬氨酸特异性半胱氨酸蛋白酶 caspase-8 抑制由 RIPK3 和 MLKL 介导的坏死性细胞死亡。事实上,缺乏 caspase-8 的小鼠在胚胎发生过程中以依赖 RIPK3 和 MLKL 的方式死亡1-3。在人类中,caspase-8 缺乏与免疫缺陷 4 或非常早发的炎症性肠病 5 相关。由 caspase-8 切割以防止体内坏死性凋亡的底物尚未确定。在这里,我们表明,由于 MLKL 依赖性坏死性凋亡,表达无催化活性的 caspase-8(C362A) 的敲入小鼠作为胚胎死亡,类似于缺乏 caspase-8 的小鼠。因此,caspase-8 必须裂解自身、其他蛋白质或两者以抑制坏死性凋亡。表达不能自我切割的 caspase-8(D212A/D218A/D225A/D387A) 的小鼠是有活力的,表达 c-FLIP 或 CYLD 蛋白的小鼠也是如此,这些蛋白已经突变以防止被 caspase-8 切割。相比之下,表达 RIPK1(D325A)(其中 caspase-8 切割位点 Asp325 已发生突变)的小鼠在妊娠中期死亡。RIPK1 失活、TNFR1 缺失或 MLKL 和 caspase-8 接头 FADD 缺失可防止胚胎致死,但仅通过 MLKL 缺失则不能。因此,RIPK1(D325A) 似乎触发了由 TNF、RIPK1 和 FADD–caspase-8 的激酶活性介导的细胞死亡。因此,含有裂解的 caspase-3 的垂死内皮细胞在 Ripk1D325A/D325A 胚胎的卵黄囊中异常丰富。杂合 Ripk1D325A/+ 细胞和小鼠是有活力的,但也比野生型细胞或小鼠更容易受到 TNF 诱导的细胞死亡的影响。
更新日期:2019-09-11
中文翻译:

caspase-8 对 RIPK1 的切割对于限制细胞凋亡和坏死性凋亡至关重要
天冬氨酸特异性半胱氨酸蛋白酶 caspase-8 抑制由 RIPK3 和 MLKL 介导的坏死性细胞死亡。事实上,缺乏 caspase-8 的小鼠在胚胎发生过程中以依赖 RIPK3 和 MLKL 的方式死亡1-3。在人类中,caspase-8 缺乏与免疫缺陷 4 或非常早发的炎症性肠病 5 相关。由 caspase-8 切割以防止体内坏死性凋亡的底物尚未确定。在这里,我们表明,由于 MLKL 依赖性坏死性凋亡,表达无催化活性的 caspase-8(C362A) 的敲入小鼠作为胚胎死亡,类似于缺乏 caspase-8 的小鼠。因此,caspase-8 必须裂解自身、其他蛋白质或两者以抑制坏死性凋亡。表达不能自我切割的 caspase-8(D212A/D218A/D225A/D387A) 的小鼠是有活力的,表达 c-FLIP 或 CYLD 蛋白的小鼠也是如此,这些蛋白已经突变以防止被 caspase-8 切割。相比之下,表达 RIPK1(D325A)(其中 caspase-8 切割位点 Asp325 已发生突变)的小鼠在妊娠中期死亡。RIPK1 失活、TNFR1 缺失或 MLKL 和 caspase-8 接头 FADD 缺失可防止胚胎致死,但仅通过 MLKL 缺失则不能。因此,RIPK1(D325A) 似乎触发了由 TNF、RIPK1 和 FADD–caspase-8 的激酶活性介导的细胞死亡。因此,含有裂解的 caspase-3 的垂死内皮细胞在 Ripk1D325A/D325A 胚胎的卵黄囊中异常丰富。杂合 Ripk1D325A/+ 细胞和小鼠是有活力的,但也比野生型细胞或小鼠更容易受到 TNF 诱导的细胞死亡的影响。

































 京公网安备 11010802027423号
京公网安备 11010802027423号