Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2019-09-11 , DOI: 10.1016/j.jcis.2019.09.034 Jing Liu , Xin Cui , Lei Xie , Jun Huang , Ling Zhang , Jifang Liu , Xiaogang Wang , Jianmei Wang , Hongbo Zeng
|
Hypothesis
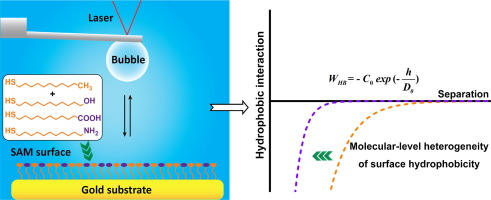
Hydrophobic interaction is crucial in various colloidal phenomena and engineering processes involving air/water/solid systems where polar and nonpolar substances usually mingle with each other. The molecular-level heterogeneity of surface hydrophobicity could potentially disorder the interfacial water molecules and thus influence the hydrophobic interaction strength, but the underlying mechanism is not completely clear.
Experiments
The hydrophobic interactions between air bubbles and self-assembled monolayer surfaces bearing methyl and different polar moieties were precisely quantified via bubble probe atomic force microscope coupled with theoretical modeling analysis.
Findings
Increasing coverage of surface polar moieties on binary-component surfaces can apparently lower surface hydrophobicity θc, but hardly affect hydrophobic interaction’s decay length D0. Changing pH has no detectable effect on hydrophobic interactions involving CH3 and CH3/OH-ended surfaces. In contrast, θc and D0 decrease monotonically with pH rising for CH3/COOH-ended surface, because high pH tends to dissociate –COOH, enhancing the surface hydrophilicity and weakening the hydrophobic interaction. For CH3/NH2-ended surface, θc and D0 decrease sequentially with pH declining, because protonation of –NH2 can lower the surface hydrophobicity and weaken the hydrophobic interaction. This work improves the fundamental understanding of hydrophobic interactions in air/water/solid systems and provides useful insights into the interfacial assembly processes in relevant engineering applications.
中文翻译:

探测表面疏水性的分子水平异质性对空气/水/固体系统中疏水相互作用的影响
假设
疏水相互作用在涉及空气/水/固体系统的各种胶体现象和工程过程中至关重要,在这些系统中,极性和非极性物质通常会相互混合。表面疏水性的分子水平异质性可能会扰乱界面水分子,从而影响疏水性相互作用强度,但其潜在机理尚不完全清楚。
实验
通过气泡探针原子力显微镜和理论建模分析,精确定量了气泡与带有甲基和不同极性部分的自组装单层表面之间的疏水相互作用。
发现
二元组分表面增加表面极性部分的覆盖范围可以明显地降低表面疏水性θ Ç,但几乎没有影响疏水性相互作用的衰减长度d 0。改变pH值对涉及CH 3和CH 3 / OH端基的疏水相互作用没有可检测的影响。与此相反,θ Ç和d 0下降单调随pH上升为CH 3 / COOH端表面,因为高pH值趋于离解-COOH,提高表面的亲水性和减弱疏水性相互作用。为CH 3 / NH 2 -ended表面,θ ÇpH下降时,D 0和D 0依次下降,这是因为–NH 2的质子化作用会降低表面疏水性并削弱疏水相互作用。这项工作提高了对空气/水/固体系统中疏水相互作用的基本理解,并为相关工程应用中的界面组装过程提供了有用的见识。

































 京公网安备 11010802027423号
京公网安备 11010802027423号