当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One-Step Synthesis of Isoindolo[2,1-a]indol-6-ones via Tandem Pd-Catalyzed Aminocarbonylation and C-H Activation.
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-09-25 , DOI: 10.1021/acs.joc.9b02008 Tomáš Čarný 1 , Martin Markovič 1, 2 , Tibor Gracza 1 , Peter Koóš 1, 2
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2019-09-25 , DOI: 10.1021/acs.joc.9b02008 Tomáš Čarný 1 , Martin Markovič 1, 2 , Tibor Gracza 1 , Peter Koóš 1, 2
Affiliation
|
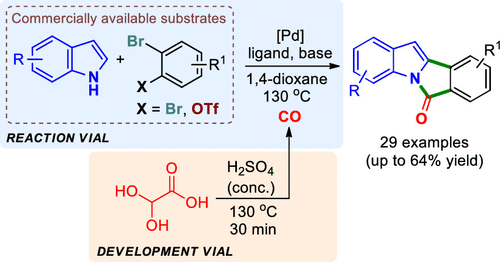
A unified catalytic system for tandem Pd-catalyzed carbonylation and C-C cross-coupling via C-H activation was designed. The proposed cascade reaction allows a facile one-step construction of a tetracyclic isoindoloindole skeleton, in which three new C-C/C-N bonds are simultaneously formed. In detail, the carbonylation of aryl dibromides with indoles and C-H activation of in situ formed N-(2'-bromoaroyl)-indole provide biologically relevant 6H-isoindolo[2,1-a]indol-6-ones from commercially available substrates. The aminocarbonylation step in the proposed tandem reaction utilizes glyoxylic acid monohydrate as an environmentally friendly CO surrogate.
中文翻译:

经由串联钯催化的氨基羰基化和CH活化一步合成Isoindolo [2,1-a] indol-6-ones。
设计了一个统一的催化体系,用于串联钯催化的羰基化和通过CH活化的CC交叉偶联。所提出的级联反应允许四环异吲哚并吲哚骨架的简便的一步构建,其中同时形成三个新的CC / CN键。详细地,芳基二溴化物与吲哚的羰基化和原位形成的N-(2'-溴芳酰基)-吲哚的CH活化从可商购的底物提供生物学上相关的6H-异吲哚并[2,1-a]吲哚-6-。建议的串联反应中的氨基羰基化步骤利用乙醛酸一水合物作为环境友好的一氧化碳替代物。
更新日期:2019-09-26
中文翻译:

经由串联钯催化的氨基羰基化和CH活化一步合成Isoindolo [2,1-a] indol-6-ones。
设计了一个统一的催化体系,用于串联钯催化的羰基化和通过CH活化的CC交叉偶联。所提出的级联反应允许四环异吲哚并吲哚骨架的简便的一步构建,其中同时形成三个新的CC / CN键。详细地,芳基二溴化物与吲哚的羰基化和原位形成的N-(2'-溴芳酰基)-吲哚的CH活化从可商购的底物提供生物学上相关的6H-异吲哚并[2,1-a]吲哚-6-。建议的串联反应中的氨基羰基化步骤利用乙醛酸一水合物作为环境友好的一氧化碳替代物。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号