当前位置:
X-MOL 学术
›
Chem. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Switch of the Rate-Determining Step of Water Oxidation by Spin-Selected Electron Transfer in Spinel Oxides
Chemistry of Materials ( IF 7.2 ) Pub Date : 2019-09-24 , DOI: 10.1021/acs.chemmater.9b02737 Shengnan Sun 1 , Yuanmiao Sun , Ye Zhou , Jingjing Shen , Daniel Mandler 2 , Ronny Neumann 1 , Zhichuan J. Xu 3, 4
Chemistry of Materials ( IF 7.2 ) Pub Date : 2019-09-24 , DOI: 10.1021/acs.chemmater.9b02737 Shengnan Sun 1 , Yuanmiao Sun , Ye Zhou , Jingjing Shen , Daniel Mandler 2 , Ronny Neumann 1 , Zhichuan J. Xu 3, 4
Affiliation
|
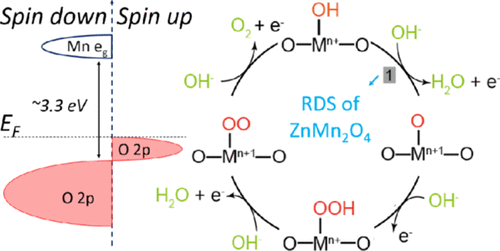
The 3d transition-metal oxides are a class of promising catalysts for the oxygen evolution reaction (OER) in alkaline media. Manganese-based spinel oxides usually do not show impressive OER performance, and their activity is generally lower than the counterparts containing cobalt. The OER activity and the electronic structure of spinel oxides ZnMn2O4, ZnCo2O4, and the cobalt-substituted ZnMn2O4 were investigated. It has been found that compared to ZnCo2O4, the lower activity of ZnMn2O4 arises from the limitation of spin-selective electron transfer and orbital symmetry restrictions between adsorption of the oxygen species onto the conduction band and the electron transfer from the conduction band to the valence band. The substitution of cobalt into ZnMn2O4 alleviates the spin incompatibility and enables the increase in OER activity.
中文翻译:

尖晶石氧化物中自旋选择电子转移的水氧化速率确定步骤的切换
3d过渡金属氧化物是一类在碱性介质中用于氧释放反应(OER)的有前途的催化剂。锰基尖晶石氧化物通常不会显示出令人印象深刻的OER性能,并且其活性通常低于含钴的尖晶石。研究了尖晶石氧化物ZnMn 2 O 4,ZnCo 2 O 4和钴取代的ZnMn 2 O 4的OER活性和电子结构。已经发现,相对于碳酸锌2 ö 4,ZnMn的较低活性2 ö 4产生自旋选择电子传递的限制和在氧物质在导带上的吸附与电子从导带到价带的转移之间的轨道对称性限制。钴取代为ZnMn 2 O 4可以减轻自旋不相容性,并可以提高OER活性。
更新日期:2019-09-25
中文翻译:

尖晶石氧化物中自旋选择电子转移的水氧化速率确定步骤的切换
3d过渡金属氧化物是一类在碱性介质中用于氧释放反应(OER)的有前途的催化剂。锰基尖晶石氧化物通常不会显示出令人印象深刻的OER性能,并且其活性通常低于含钴的尖晶石。研究了尖晶石氧化物ZnMn 2 O 4,ZnCo 2 O 4和钴取代的ZnMn 2 O 4的OER活性和电子结构。已经发现,相对于碳酸锌2 ö 4,ZnMn的较低活性2 ö 4产生自旋选择电子传递的限制和在氧物质在导带上的吸附与电子从导带到价带的转移之间的轨道对称性限制。钴取代为ZnMn 2 O 4可以减轻自旋不相容性,并可以提高OER活性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号