当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure of a Ferryl Mimic in the Archetypal Iron(II)- and 2-(Oxo)-glutarate-Dependent Dioxygenase, TauD.
Biochemistry ( IF 2.9 ) Pub Date : 2019-10-02 , DOI: 10.1021/acs.biochem.9b00598 Katherine M. Davis , Madison Altmyer , Ryan J. Martinie , Irene Schaperdoth , Carsten Krebs , J. Martin Bollinger , Amie K. Boal
Biochemistry ( IF 2.9 ) Pub Date : 2019-10-02 , DOI: 10.1021/acs.biochem.9b00598 Katherine M. Davis , Madison Altmyer , Ryan J. Martinie , Irene Schaperdoth , Carsten Krebs , J. Martin Bollinger , Amie K. Boal
|
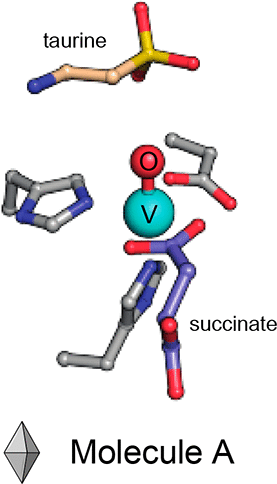
Iron(II)- and 2-(oxo)-glutarate-dependent (Fe/2OG) oxygenases catalyze a diverse array of oxidation reactions via a common iron(IV)-oxo (ferryl) intermediate. Although the intermediate has been characterized spectroscopically, its short lifetime has precluded crystallograhic characterization. In solution, the ferryl was first observed directly in the archetypal Fe/2OG hydroxylase, taurine:2OG dioxygenase (TauD). Here, we substitute the iron cofactor of TauD with the stable vanadium(IV)-oxo (vanadyl) ion to obtain crystal structures mimicking the key ferryl complex. Intriguingly, whereas the structure of the TauD·(VIV-oxo)·succinate·taurine complex exhibits the expected orientation of the V≡O bond—trans to the His255 ligand and toward the C–H bond to be cleaved, in what has been termed the in-line configuration—the TauD·(VIV-oxo) binary complex is best modeled with its oxo ligand trans to Asp101. This off-line-like configuration is similar to one recently posited as a means to avoid hydroxylation in Fe/2OG enzymes that direct other outcomes, though neither has been visualized in an Fe/2OG structure to date. Whereas an off-line (trans to the proximal His) or off-line-like (trans to the carboxylate ligand) ferryl is unlikely to be important in the hydroxylation reaction of TauD, the observation that the ferryl may deviate from an in-line orientation in the absence of the primary substrate may explain the enzyme’s mysterious self-hydroxylation behavior, should the oxo ligand lie trans to His99. This finding reinforces the potential for analogous functional off-line oxo configurations in halogenases, desaturases, and/or cyclases.
中文翻译:

原型铁(II)-和2-(Oxo)-戊二酸依赖性双加氧酶TauD中的轮渡模拟物的结构。
依赖于铁(II)-和2-(氧代)-谷氨酸的(Fe / 2OG)加氧酶可通过常见的铁(IV)-氧代(轮式)中间体催化各种氧化反应。尽管该中间体已通过光谱表征,但其使用寿命短,无法进行结晶学表征。在溶液中,首先在原型Fe / 2OG羟化酶,牛磺酸:2OG双加氧酶(TauD)中直接观察到小轮。在这里,我们用稳定的钒(IV)-氧代(钒基)离子代替了TauD的铁辅因子,从而获得了模拟关键小轮配合物的晶体结构。有趣的是,而TAUD的结构·(V IV -氧)·琥珀酸酯·牛磺酸复合物表现出V≡O成键的预期定向反称为直链构型的TauD·(V IV -oxo )二元配合物最好是通过将其oxo配体反式转化为Asp101来形成的。这种离线样的构型类似于最近被定位为避免指导其他结果的Fe / 2OG酶中羟基化的一种构型,尽管迄今为止在Fe / 2OG结构中都没有观察到这种构型。脱机(反接至近端His)或脱机式(反接对于羧酸盐配体),在TauD的羟基化反应中,亚铁不太可能重要。如果没有主要底物,则亚铁可能偏离在线方向,这一观察结果可以解释该酶的神秘自羟基化行为。氧代配体位于反义到His99。这一发现增强了卤化酶,去饱和酶和/或环化酶中类似的功能性离线羰基合成构型的潜力。
更新日期:2019-10-03
中文翻译:

原型铁(II)-和2-(Oxo)-戊二酸依赖性双加氧酶TauD中的轮渡模拟物的结构。
依赖于铁(II)-和2-(氧代)-谷氨酸的(Fe / 2OG)加氧酶可通过常见的铁(IV)-氧代(轮式)中间体催化各种氧化反应。尽管该中间体已通过光谱表征,但其使用寿命短,无法进行结晶学表征。在溶液中,首先在原型Fe / 2OG羟化酶,牛磺酸:2OG双加氧酶(TauD)中直接观察到小轮。在这里,我们用稳定的钒(IV)-氧代(钒基)离子代替了TauD的铁辅因子,从而获得了模拟关键小轮配合物的晶体结构。有趣的是,而TAUD的结构·(V IV -氧)·琥珀酸酯·牛磺酸复合物表现出V≡O成键的预期定向反称为直链构型的TauD·(V IV -oxo )二元配合物最好是通过将其oxo配体反式转化为Asp101来形成的。这种离线样的构型类似于最近被定位为避免指导其他结果的Fe / 2OG酶中羟基化的一种构型,尽管迄今为止在Fe / 2OG结构中都没有观察到这种构型。脱机(反接至近端His)或脱机式(反接对于羧酸盐配体),在TauD的羟基化反应中,亚铁不太可能重要。如果没有主要底物,则亚铁可能偏离在线方向,这一观察结果可以解释该酶的神秘自羟基化行为。氧代配体位于反义到His99。这一发现增强了卤化酶,去饱和酶和/或环化酶中类似的功能性离线羰基合成构型的潜力。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号