当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
NiAl2O4 Spinel Supported Pt Catalyst: High Performance and Origin in Aqueous-Phase Reforming of Methanol
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-09-26 , DOI: 10.1021/acscatal.9b02243 Didi Li,Yi Li,Xiaohui Liu,Yong Guo,Chih-Wen Pao,Jeng-Lung Chen,Yongfeng Hu,Yanqin Wang
ACS Catalysis ( IF 11.3 ) Pub Date : 2019-09-26 , DOI: 10.1021/acscatal.9b02243 Didi Li,Yi Li,Xiaohui Liu,Yong Guo,Chih-Wen Pao,Jeng-Lung Chen,Yongfeng Hu,Yanqin Wang
|
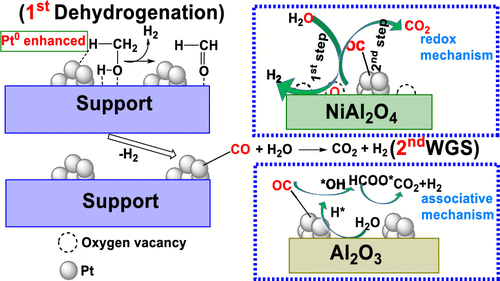
The production of hydrogen from the aqueous-phase reforming (APR) of oxygenated hydrocarbons is promising. Herein, the performances of Pt loaded on NiAl2O4 spinel and γ-Al2O3 were investigated in the APR of methanol. The conversion of methanol and the yield of hydrogen over Pt/NiAl2O4 reached 99.9% and 95.7%, respectively. In comparison with Pt/γ-Al2O3 catalyst (26.5% and 23.3%, respectively), these values were enhanced by 4-fold. More importantly, Pt/NiAl2O4 had high stability with only 10% loss of its initial conversion after 600 h on stream. In situ diffuse reflectance infrared Fourier transform spectra (DRIFTS) of the APR of methanol revealed that the reaction underwent the dehydrogenation of methanol and the sequential water–gas shift (WGS) reaction. These two reactions were then investigated independently, in which Pt/NiAl2O4 showed more efficient performance than Pt/γ-Al2O3. Intensive characterization methods revealed that the chemical state of Pt played a pivotal role in the dehydrogenation of methanol to generate the adsorbed CO intermediate. For Pt/NiAl2O4 catalyst, the reduction of PtOx to metallic state Pt was easier because of the presence of the oxygen vacancy, leading to the higher catalytic performance in the dehydrogenation of methanol. Further studies with in situ DRIFTS-MS of WGS demonstrated a redox mechanism over Pt/NiAl2O4 catalyst, which was different from the associative route that occurred over Pt/γ-Al2O3 and made the WGS reaction faster. The addition of Ni (NiAl2O4 spinel) creates oxygen vacancies, giving WGS which underwent a redox route. This work presents the deep understanding into the pathway and mechanism in the APR of methanol and is expected to have important implications for the future development of APR catalysts.
中文翻译:

NiAl 2 O 4尖晶石负载的Pt催化剂:甲醇水相重整的高性能和起源
由含氧烃的水相重整(APR)生产氢很有希望。这里,装载在的NiAl的Pt的性能2 Ò 4尖晶石和在γ-Al 2 ö 3在甲醇中的APR进行了调查。Pt / NiAl 2 O 4上甲醇的转化率和氢气的收率分别达到99.9%和95.7%。在有Pt /γ-Al系比较2 ö 3(分别为26.5%和23.3%,)催化剂,这些值是由4倍增强。更重要的是,Pt / NiAl 2 O 4具有高稳定性,在运行600小时后其初始转化率仅损失10%。原位甲醇的APR的漫反射红外傅里叶变换光谱(DRIFTS)表明,反应进行了甲醇的脱氢反应和连续的水煤气变换(WGS)反应。然后,这两个反应均独立地研究,其中的Pt /的NiAl 2 ö 4显示出比的Pt /γ-Al系更高效的性能2 ö 3。强化表征方法表明,Pt的化学状态在甲醇脱氢以生成吸附的CO中间体方面起着关键作用。对于Pt / NiAl 2 O 4催化剂,PtO x的还原由于氧空位的存在,使Pt转变为金属态更容易,从而在甲醇脱氢中具有更高的催化性能。与进一步的研究原位WGS的DRIFTS-MS证实在Pt / NiAl金属的氧化还原机构2 ö 4催化剂,将其从发生在Pt /γ-Al系缔合的路线不同的2 ö 3和取得的WGS反应更快。Ni(NiAl 2 O 4尖晶石)的添加会产生氧空位,从而使WGS经历了氧化还原途径。这项工作提供了对甲醇APR的途径和机理的深刻理解,并有望对APR催化剂的未来发展产生重要影响。
更新日期:2019-09-26
中文翻译:

NiAl 2 O 4尖晶石负载的Pt催化剂:甲醇水相重整的高性能和起源
由含氧烃的水相重整(APR)生产氢很有希望。这里,装载在的NiAl的Pt的性能2 Ò 4尖晶石和在γ-Al 2 ö 3在甲醇中的APR进行了调查。Pt / NiAl 2 O 4上甲醇的转化率和氢气的收率分别达到99.9%和95.7%。在有Pt /γ-Al系比较2 ö 3(分别为26.5%和23.3%,)催化剂,这些值是由4倍增强。更重要的是,Pt / NiAl 2 O 4具有高稳定性,在运行600小时后其初始转化率仅损失10%。原位甲醇的APR的漫反射红外傅里叶变换光谱(DRIFTS)表明,反应进行了甲醇的脱氢反应和连续的水煤气变换(WGS)反应。然后,这两个反应均独立地研究,其中的Pt /的NiAl 2 ö 4显示出比的Pt /γ-Al系更高效的性能2 ö 3。强化表征方法表明,Pt的化学状态在甲醇脱氢以生成吸附的CO中间体方面起着关键作用。对于Pt / NiAl 2 O 4催化剂,PtO x的还原由于氧空位的存在,使Pt转变为金属态更容易,从而在甲醇脱氢中具有更高的催化性能。与进一步的研究原位WGS的DRIFTS-MS证实在Pt / NiAl金属的氧化还原机构2 ö 4催化剂,将其从发生在Pt /γ-Al系缔合的路线不同的2 ö 3和取得的WGS反应更快。Ni(NiAl 2 O 4尖晶石)的添加会产生氧空位,从而使WGS经历了氧化还原途径。这项工作提供了对甲醇APR的途径和机理的深刻理解,并有望对APR催化剂的未来发展产生重要影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号