Chemical Engineering Journal ( IF 13.3 ) Pub Date : 2019-09-11 , DOI: 10.1016/j.cej.2019.122807 Hongjie Cui , Xinlei Li , Hui Chen , Xiongyi Gu , Zhenmin Cheng , Zhiming Zhou
|
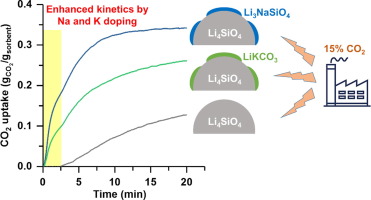
Li4SiO4 is considered as one of the most potential high-temperature sorbent materials for CO2 capture from the flue gas of fossil fuel-fired power plants. However, it usually suffers from slow kinetics at low CO2 concentrations. In this work, a series of Na- and K-doped Li4SiO4-based sorbents with Na/Si (or K/Si) atomic ratios of 0.05, 0.1 and 0.2 were prepared by a citrate sol-gel method. The prepared materials were characterized by various techniques, which revealed the presence of Li3NaSiO4 (for Na-doped sorbents) and LiKCO3 (for K-doped sorbents) on the surface of Li4SiO4 particles. All Na- and K-doped Li4SiO4 showed greatly enhanced CO2 sorption rates and capacities in 15% CO2 as compared to the heteroatom-free Li4SiO4, and in particular, the Na-doped sorbents had the fastest rates. The improved performance of K-doped Li4SiO4 resulted from the formation of eutectic Li2CO3-K2CO3 molten carbonates during CO2 sorption, while for Na-doped Li4SiO4, both the carbonation of Li3NaSiO4 and the presence of eutectic Li2CO3-Na2CO3 molten salts contributed to the improvement. In addition, both Na- and K-doped Li4SiO4 exhibited incomplete regeneration during cyclic operation, but the latter had better stability. In this context, we deliberately prepared a Na,K-codoped Li4SiO4-based sorbent with a Na:K:Si atomic ratio of 0.1:0.1:1, which showed fast kinetics and good cyclic stability. During 30 consecutive sorption-regeneration cycles (10 min of sorption in 15% CO2 at 650 °C, 5 min of regeneration in pure N2 at 750 °C), the Na,K-codoped Li4SiO4 presented a stable CO2 capture capacity of 0.22 gCO2/gsorbent after the first 10 cycles.
中文翻译:

溶胶凝胶衍生,Na / K掺杂的Li 4 SiO 4基CO 2吸附剂,在高温下具有快速动力学
Li 4 SiO 4被认为是从化石燃料发电厂的烟道气中捕获CO 2最具潜力的高温吸附剂材料之一。但是,它通常在低CO 2浓度下动力学缓慢。在这项工作中,通过柠檬酸溶胶-凝胶法制备了一系列Na / Si(或K / Si)原子比为0.05、0.1和0.2的Na和K掺杂的Li 4 SiO 4基吸附剂。所制备的材料通过各种技术进行表征,表明在Li 4 SiO的表面上存在Li 3 NaSiO 4(用于掺杂Na的吸附剂)和LiKCO 3(用于掺杂K的吸附剂)。4个粒子。与不含杂原子的Li 4 SiO 4相比,所有Na和K掺杂的Li 4 SiO 4均表现出大大提高的CO 2吸附率和在15%CO 2中的容量,尤其是Na掺杂的吸附剂具有最快的吸附速率。 。K掺杂的Li 4 SiO 4的性能改善是由于在CO 2吸附过程中形成了共晶的Li 2 CO 3 -K 2 CO 3熔融碳酸盐,而对于Na掺杂的Li 4 SiO 4,Li 3 NaSiO均被碳化。如图4所示,共晶Li 2 CO 3 -Na 2 CO 3熔融盐的存在有助于改善。另外,Na和K掺杂的Li 4 SiO 4在循环操作中均表现出不完全再生,但后者具有更好的稳定性。在此背景下,我们特意制备了Na :K:Si:0.1:0.1:1的Na,K掺杂的Li 4 SiO 4基吸附剂,该吸附剂显示出快速的动力学和良好的循环稳定性。在30个连续的吸附-再生循环中(在650°C的15%CO 2中吸附10分钟,在750°C的纯N 2中再生5分钟),掺有Na,K的Li 4在前10个循环后,SiO 4的稳定CO 2捕集能力为0.22 g CO2 / g吸附剂。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号