Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Evaluation Approach of Cell Viability Based on Cell Detachment Assay in a Single-Channel Integrated Microfluidic Chip.
ACS Sensors ( IF 8.2 ) Pub Date : 2019-09-24 , DOI: 10.1021/acssensors.9b01061 Mingji Wei , Rongbiao Zhang , Fei Zhang , Yecheng Zhang , Guoxiao Li , Renjie Miao , Shihe Shao
ACS Sensors ( IF 8.2 ) Pub Date : 2019-09-24 , DOI: 10.1021/acssensors.9b01061 Mingji Wei , Rongbiao Zhang , Fei Zhang , Yecheng Zhang , Guoxiao Li , Renjie Miao , Shihe Shao
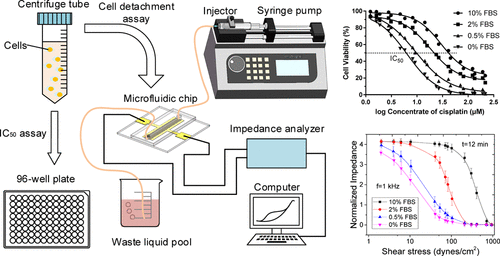
|
Due to the heterogeneity of cancer cell populations, the traditional evaluation approach of cell viability based on the cell counting assay is quite inaccurate for the dose–response test of anticancer drugs, cell toxicology assays, and other biochemical stimulations. In this paper, an evaluation approach of cell viability based on the cell detachment assay in a single-channel integrated microfluidic chip is proposed to improve the accuracy of cell viability assessment. The electrodes are coated by fibronectin for specific cell adhesion, and it is biologically significant to study the cell detachment assay in vitro. The maximum number of cells that can be detected by this sensor is about 105 cells (overgrowing), while the minimum is about 100 cells. This method is calibrated with the half-maximal inhibitory concentration assay, and the results show that the cell viability calculated by adhesion strength is more accurate than that evaluated using the cell counting assay. Meanwhile, the shear rate is transformed into shear stress for the comparability among the results in other papers. The most sensitive frequency is also determined as 1 kHz according to normalized impedance. Besides, the impedance of cell adhesion affected by different shear stresses is monitored to study the optimized plan for long-term culture of cells in the integrated microfluidic chip prepared for the cell detachment assay. Adhesion strength τ25, which is the magnitude of shear stress needed to detach 75% of cell population, is introduced to describe the cell adhesion forces. It is calculated and normalized based on the cell detachment assay to evaluate cell viability. The relative errors of the cell detachment method compared with those of the cell counting method decrease by 0.637 (0% FBS), 0.586 (0.5% FBS), and 0.342 (2% FBS).
中文翻译:

基于细胞分离分析的单通道集成微流控芯片中细胞活力的评估方法。
由于癌细胞群体的异质性,基于细胞计数测定法的传统细胞活力评估方法对于抗癌药的剂量反应测试,细胞毒理学测定法和其他生化刺激非常不准确。本文提出了一种基于细胞分离分析的单通道集成微流控芯片细胞活力评估方法,以提高细胞活力评估的准确性。电极被纤连蛋白包被,可特异性粘附细胞,在体外研究细胞脱离测定具有生物学意义。该传感器可检测的最大细胞数约为10 5个单元格(过度生长),而最小值约为100个单元格。该方法用最大抑制浓度的一半进行校准,结果表明,通过粘附强度计算的细胞活力比使用细胞计数分析评估的细胞活力更为准确。同时,为了与其他论文进行比较,将剪切速率转换为剪切应力。根据归一化阻抗,也将最敏感的频率确定为1 kHz。此外,监测受不同剪切应力影响的细胞粘附的阻抗,以研究在用于细胞分离测定的集成微流控芯片中长期培养细胞的优化计划。粘合强度τ 25引入,它是分离75%的细胞群体所需的剪切应力的大小,用来描述细胞的粘附力。基于细胞分离测定法计算并归一化,以评估细胞活力。与细胞计数方法相比,细胞分离方法的相对误差降低了0.637(0%FBS),0.586(0.5%FBS)和0.342(2%FBS)。
更新日期:2019-09-25
中文翻译:

基于细胞分离分析的单通道集成微流控芯片中细胞活力的评估方法。
由于癌细胞群体的异质性,基于细胞计数测定法的传统细胞活力评估方法对于抗癌药的剂量反应测试,细胞毒理学测定法和其他生化刺激非常不准确。本文提出了一种基于细胞分离分析的单通道集成微流控芯片细胞活力评估方法,以提高细胞活力评估的准确性。电极被纤连蛋白包被,可特异性粘附细胞,在体外研究细胞脱离测定具有生物学意义。该传感器可检测的最大细胞数约为10 5个单元格(过度生长),而最小值约为100个单元格。该方法用最大抑制浓度的一半进行校准,结果表明,通过粘附强度计算的细胞活力比使用细胞计数分析评估的细胞活力更为准确。同时,为了与其他论文进行比较,将剪切速率转换为剪切应力。根据归一化阻抗,也将最敏感的频率确定为1 kHz。此外,监测受不同剪切应力影响的细胞粘附的阻抗,以研究在用于细胞分离测定的集成微流控芯片中长期培养细胞的优化计划。粘合强度τ 25引入,它是分离75%的细胞群体所需的剪切应力的大小,用来描述细胞的粘附力。基于细胞分离测定法计算并归一化,以评估细胞活力。与细胞计数方法相比,细胞分离方法的相对误差降低了0.637(0%FBS),0.586(0.5%FBS)和0.342(2%FBS)。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号