Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Aberrant Regulation of RAD51 Promotes Resistance of Neoadjuvant Endocrine Therapy in ER-positive Breast Cancer.
Scientific Reports ( IF 3.8 ) Pub Date : 2019-09-10 , DOI: 10.1038/s41598-019-49373-w Yan Jia 1 , Yueshuai Song 2 , Guolei Dong 1 , Chunfang Hao 1 , Weipeng Zhao 1 , Shufen Li 1 , Zhongsheng Tong 1
Scientific Reports ( IF 3.8 ) Pub Date : 2019-09-10 , DOI: 10.1038/s41598-019-49373-w Yan Jia 1 , Yueshuai Song 2 , Guolei Dong 1 , Chunfang Hao 1 , Weipeng Zhao 1 , Shufen Li 1 , Zhongsheng Tong 1
Affiliation
|
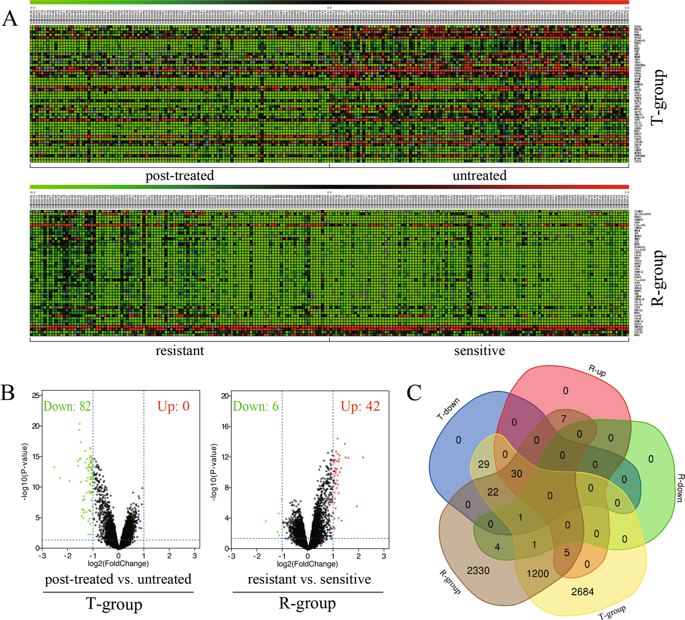
Breast cancer is one of the most common malignant cancers affecting females. Estrogen receptor (ER)-positive breast cancer is responsive to endocrine therapy. Although current therapies offer favorable prospects for improving survival, the development of resistance remains a severe problem. In this study, we explored the resistance mechanisms of ER-positive breast cancer to neoadjuvant endocrine therapy. Microarray data of GSE87411 contained 109 pairs of samples from Z1031 trial, including untreated samples and post-treated samples with neoadjuvant aromatase inhibitor (AI) therapy. The differentially expressed genes (DEGs) were obtained from two different comparisons: untreated samples versus post-treated samples with AIs, and post-treated samples sensitive versus resistant to AIs. Multiple bioinformatic methods were applied to evaluate biological function, protein-protein network and potential binding between target protein and aromatase inhibitor. Then, regulation of gene expression, DNA methylation and clinicopathological factors of breast cancer were further analyzed with TCGA data. From GSE87411 dataset, 30 overlapped DEGs were identified. Cell division was found to be the main function of overlapped DEGs by functional enrichment and gene ontology (GO) analysis. RAD51 recombinase (RAD51), a key protein of homologous recombination, was detected to interact with BReast CAncer genes 2 (BRCA2). Moreover, according to the docking simulation, RAD51 might potentially bind to AIs. Overexpressed RAD51 was associated with hypermethylation of BRCA2, resistance to AIs and poor overall survival of patients with ER-positive breast cancer. Furthermore, RAD51 was found to be a better indicator than MKI67 for predicting resistance in neoadjuvant setting. The results indicated that methylation of BRCA2 led to incomplete suppression on RAD51, which caused an increased expression of RAD51, subsequently AI-resistance and poor prognosis in ER-positive breast cancer. RAD51 could be a new candidate used as a predicative marker and therapeutic target in neoadjuvant endocrine treatment.
中文翻译:

RAD51的异常调节可促进ER阳性乳腺癌新辅助内分泌治疗的耐药性。
乳腺癌是影响女性的最常见的恶性肿瘤之一。雌激素受体(ER)阳性的乳腺癌对内分泌治疗有反应。尽管目前的疗法为改善存活率提供了有利的前景,但耐药性的发展仍然是一个严重的问题。在这项研究中,我们探讨了ER阳性乳腺癌对新辅助内分泌治疗的耐药机制。GSE87411的微阵列数据包含来自Z1031试验的109对样品,包括未经处理的样品和采用新辅助芳香化酶抑制剂(AI)治疗的后处理样品。从两个不同的比较中获得差异表达的基因(DEG):未经处理的样品与具有AI的后处理样品,以及对AI敏感或具有抗性的后处理样品。多种生物信息学方法被用于评估生物学功能,蛋白质-蛋白质网络以及目标蛋白质和芳香化酶抑制剂之间的潜在结合。然后,利用TCGA数据进一步分析乳腺癌的基因表达,DNA甲基化和临床病理因素。从GSE87411数据集中,鉴定出30个重叠的DEG。通过功能富集和基因本体论(GO)分析,发现细胞分裂是重叠DEG的主要功能。RAD51重组酶(RAD51)是同源重组的关键蛋白,被发现与BReast CAncer基因2(BRCA2)相互作用。此外,根据对接模拟,RAD51可能会绑定到AI。过度表达的RAD51与BRCA2的甲基化有关,ER阳性乳腺癌患者对AI的耐药性和较差的总体生存率。此外,发现RAD51是比MKI67更好的指示剂,可以预测新辅助治疗中的耐药性。结果表明,BRCA2的甲基化导致对RAD51的不完全抑制,从而导致RAD51的表达增加,继而对ER阳性乳腺癌的AI耐药性和预后不良。RAD51可能是新辅助内分泌治疗中用作预测标记和治疗靶标的新候选药物。随后,ER阳性乳腺癌的AI耐药性和预后不良。RAD51可能是新辅助内分泌治疗中用作预测标记和治疗靶标的新候选药物。随后,ER阳性乳腺癌的AI耐药性和预后不良。RAD51可能是新辅助内分泌治疗中用作预测标记和治疗靶标的新候选药物。
更新日期:2019-09-10
中文翻译:

RAD51的异常调节可促进ER阳性乳腺癌新辅助内分泌治疗的耐药性。
乳腺癌是影响女性的最常见的恶性肿瘤之一。雌激素受体(ER)阳性的乳腺癌对内分泌治疗有反应。尽管目前的疗法为改善存活率提供了有利的前景,但耐药性的发展仍然是一个严重的问题。在这项研究中,我们探讨了ER阳性乳腺癌对新辅助内分泌治疗的耐药机制。GSE87411的微阵列数据包含来自Z1031试验的109对样品,包括未经处理的样品和采用新辅助芳香化酶抑制剂(AI)治疗的后处理样品。从两个不同的比较中获得差异表达的基因(DEG):未经处理的样品与具有AI的后处理样品,以及对AI敏感或具有抗性的后处理样品。多种生物信息学方法被用于评估生物学功能,蛋白质-蛋白质网络以及目标蛋白质和芳香化酶抑制剂之间的潜在结合。然后,利用TCGA数据进一步分析乳腺癌的基因表达,DNA甲基化和临床病理因素。从GSE87411数据集中,鉴定出30个重叠的DEG。通过功能富集和基因本体论(GO)分析,发现细胞分裂是重叠DEG的主要功能。RAD51重组酶(RAD51)是同源重组的关键蛋白,被发现与BReast CAncer基因2(BRCA2)相互作用。此外,根据对接模拟,RAD51可能会绑定到AI。过度表达的RAD51与BRCA2的甲基化有关,ER阳性乳腺癌患者对AI的耐药性和较差的总体生存率。此外,发现RAD51是比MKI67更好的指示剂,可以预测新辅助治疗中的耐药性。结果表明,BRCA2的甲基化导致对RAD51的不完全抑制,从而导致RAD51的表达增加,继而对ER阳性乳腺癌的AI耐药性和预后不良。RAD51可能是新辅助内分泌治疗中用作预测标记和治疗靶标的新候选药物。随后,ER阳性乳腺癌的AI耐药性和预后不良。RAD51可能是新辅助内分泌治疗中用作预测标记和治疗靶标的新候选药物。随后,ER阳性乳腺癌的AI耐药性和预后不良。RAD51可能是新辅助内分泌治疗中用作预测标记和治疗靶标的新候选药物。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号