Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2019-09-10 , DOI: 10.1016/j.bioorg.2019.103260 Fang Yang 1 , Liang-Zhentian Yu 2 , Peng-Cheng Diao 1 , Xie-Er Jian 1 , Ming-Feng Zhou 1 , Cui-Shan Jiang 1 , Wen-Wei You 1 , Wei-Feng Ma 2 , Pei-Liang Zhao 1
|
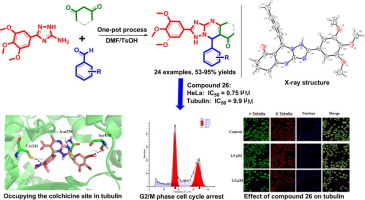
As restricted CA-4 analogues, a novel series of [1,2,4]triazolo[1,5-a]pyrimidines possessing 3,4,5-trimethoxylphenyl groups has been achieved successfully via an efficient one-pot three-component reaction of 3-(3,4,5-trimethoxyphenyl)-1H-1,2,4-triazol-5-amine, 1,3-dicarbonyl compounds and aldehydes. Initial biological evaluation demonstrated some of target compounds displayed potent antitumor activity in vitro against three cancer cell lines. Among them, the most highly active analogue 26 inhibited the growth of HeLa, and A549 cell lines with IC50 values at 0.75, and 1.02 μM, respectively, indicating excellent selectivity over non-tumoural cell line HEK-293 (IC50 = 29.94 μM) which suggested that the target compounds might possess a high safety index. Moreover, cell cycle analysis illustrated that the analogue 26 significantly induced HeLa cells arrest in G2/M phase, meanwhile the compound could dramatically affect cell morphology and microtubule networks. In addition, compound 28 exhibited potent anti-tubulin activity with IC50 values of 9.90 μM, and molecular docking studies revealed the analogue occupied the colchicine-binding site of tubulin. These observations suggest that [1,2,4]triazolo[1,5-a]pyrimidines represent a new class of tubulin polymerization inhibitors and well worth further investigation aiming to generate potential anticancer agents.
中文翻译:

新型[1,2,4]三唑并[1,5-a]嘧啶衍生物作为有效的抗微管蛋白剂:设计,多组分合成和抗增殖活性。
作为受限制的CA-4类似物,通过有效的一锅三组分反应成功地获得了具有3,4,5-三甲氧基苯基的一系列新的[1,2,4]三唑并[1,5- a ]嘧啶系列3-(3,4,5-三甲氧基苯基)-1H-1,2,4-三唑-5-胺,1,3-二羰基化合物和醛。初步的生物学评估表明,某些目标化合物在体外对三种癌细胞显示出有效的抗肿瘤活性。其中,活性最高的类似物26抑制HeLa和A549细胞株的生长,其IC 50值分别为0.75和1.02μM,这表明与非肿瘤细胞株HEK-293(IC 50 = 29.94μM),这表明目标化合物可能具有较高的安全指数。此外,细胞周期分析表明,类似物26明显诱导HeLa细胞停滞在G2 / M期,同时该化合物可显着影响细胞形态和微管网络。此外,化合物28表现出有效的抗微管蛋白活性,IC 50值为9.90μM,分子对接研究表明,该类似物占据了微管蛋白的秋水仙碱结合位点。这些观察结果表明,[1,2,4]三唑并[1,5- a ]嘧啶代表了一类新的微管蛋白聚合抑制剂,非常值得进一步研究以产生潜在的抗癌药。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号