Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Redox Mediator: A New Strategy in Designing Cathode for Prompting Redox Process of Li–S Batteries
Advanced Science ( IF 14.3 ) Pub Date : 2019-09-10 , DOI: 10.1002/advs.201900958 Xian Wu 1 , Nannan Liu 1 , Bin Guan 1 , Yue Qiu 1 , Maoxu Wang 1 , Junhan Cheng 1 , Da Tian 1 , Lishuang Fan 1, 2 , Naiqing Zhang 1, 2 , Kening Sun 1, 2
Advanced Science ( IF 14.3 ) Pub Date : 2019-09-10 , DOI: 10.1002/advs.201900958 Xian Wu 1 , Nannan Liu 1 , Bin Guan 1 , Yue Qiu 1 , Maoxu Wang 1 , Junhan Cheng 1 , Da Tian 1 , Lishuang Fan 1, 2 , Naiqing Zhang 1, 2 , Kening Sun 1, 2
Affiliation
|
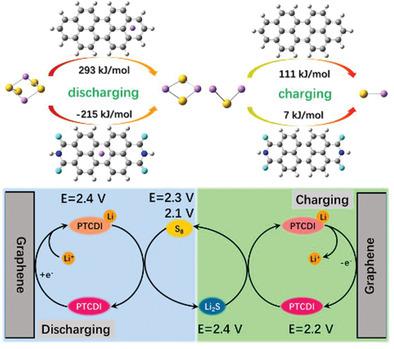
The multistep redox reactions of lithium–sulfur batteries involve undesirably complex transformation between sulfur and Li2S, and it is tough to spontaneously fragmentate polysulfides into shorter chains Li2S originating from the sluggish redox kinetics of soluble polysulfide intermediates, causing serious polarization and consumption of sulfur. In this work, 3,4,9,10‐perylenetetracarboxylic diimide (PTCDI)/G is employed as sulfur host to accelerate the conversion process between polysulfides and sulfur, which could facilitate the process of both charging and discharging. Moreover, PTCDI has strong adsorption capacity with polysulfides to restrain shuttle effect, resulting in promotional kinetics and cycle stability. A high initial capacity of 1496 mAh g−1 at 0.05 C and slight capacity decay of 0.009% per cycle at 5 C over 1500 cycles can be achieved. Moreover, the cathode could also achieve a high energy efficiency over 85% at 0.5 C. This research extends the knowledge into an original domain for designing high‐performance host materials.
中文翻译:

氧化还原介体:促进锂硫电池氧化还原过程的正极设计新策略
锂硫电池的多步氧化还原反应涉及硫和Li 2 S之间的复杂转化,并且由于可溶性多硫化物中间体氧化还原动力学缓慢,很难自发地将多硫化物裂解成较短链的Li 2 S,从而导致严重的极化和消耗硫磺。在这项工作中,采用3,4,9,10-苝四甲酰二亚胺(PTCDI)/G作为硫主体来加速多硫化物和硫之间的转化过程,从而促进充电和放电过程。此外,PTCDI对多硫化物具有很强的吸附能力,可以抑制穿梭效应,从而提高动力学和循环稳定性。可以实现在0.05C下1496mAh g -1的高初始容量以及在5C下超过1500次循环时每次循环0.009%的轻微容量衰减。此外,阴极在0.5 C时还可以实现超过85%的高能量效率。这项研究将知识扩展到设计高性能主体材料的原始领域。
更新日期:2019-09-10
中文翻译:

氧化还原介体:促进锂硫电池氧化还原过程的正极设计新策略
锂硫电池的多步氧化还原反应涉及硫和Li 2 S之间的复杂转化,并且由于可溶性多硫化物中间体氧化还原动力学缓慢,很难自发地将多硫化物裂解成较短链的Li 2 S,从而导致严重的极化和消耗硫磺。在这项工作中,采用3,4,9,10-苝四甲酰二亚胺(PTCDI)/G作为硫主体来加速多硫化物和硫之间的转化过程,从而促进充电和放电过程。此外,PTCDI对多硫化物具有很强的吸附能力,可以抑制穿梭效应,从而提高动力学和循环稳定性。可以实现在0.05C下1496mAh g -1的高初始容量以及在5C下超过1500次循环时每次循环0.009%的轻微容量衰减。此外,阴极在0.5 C时还可以实现超过85%的高能量效率。这项研究将知识扩展到设计高性能主体材料的原始领域。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号