当前位置:
X-MOL 学术
›
Green Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Achmatowicz rearrangement enables hydrogenolysis-free gas-phase synthesis of pentane-1,2,5-triol from furfuryl alcohol
Green Chemistry ( IF 9.3 ) Pub Date : 2019-09-10 , DOI: 10.1039/c9gc02888a Svilen P. Simeonov 1, 2, 3, 4 , Hristina I. Lazarova 1, 2, 3, 4 , Maya K. Marinova 1, 2, 3, 4 , Margarita D. Popova 1, 2, 3, 4
Green Chemistry ( IF 9.3 ) Pub Date : 2019-09-10 , DOI: 10.1039/c9gc02888a Svilen P. Simeonov 1, 2, 3, 4 , Hristina I. Lazarova 1, 2, 3, 4 , Maya K. Marinova 1, 2, 3, 4 , Margarita D. Popova 1, 2, 3, 4
Affiliation
|
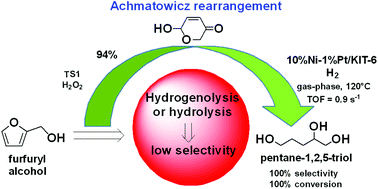
Highly efficient synthesis of pentane-1,2,5 triol, a promising member of biorenewable C5 alcohols, has been achieved by gas-phase hydrogenation of the Achmatowicz intermediate derived from furfuryl alcohol. The hydrogenation was carried out on monocomponent or bicomponent Ni and/or Pt modified mesoporous silica catalysts. The process features the absence of hydrogenolysis of the furan ring and rendered 100% selectivity together with additional green chemistry benefits, such as mild and simpler solvent free technology that operates at atmospheric pressure. The bicomponent Ni/Pt modified mesoporous silica catalysts exhibited the highest catalytic activity, with 10Ni1Pt/KIT-6 being the most active, providing up to 100% conversion.
中文翻译:

通过Achmatowicz重排,可以从糠醇中无氢解气相合成戊烷1,2,5-三醇
通过气相氢化衍生自糠醇的Achmatowicz中间体,已实现了戊烷1,2,5三醇(一种可生物再生的C5醇)的高效合成。在单组分或双组分Ni和/或Pt改性的介孔二氧化硅催化剂上进行氢化。该工艺的特点是不存在呋喃环的氢解反应,并具有100%的选择性,并具有其他绿色化学优点,例如在大气压下运行的温和且简单的无溶剂技术。双组分Ni / Pt改性介孔二氧化硅催化剂表现出最高的催化活性,其中10Ni1Pt / KIT-6最具活性,可提供高达100%的转化率。
更新日期:2019-10-14
中文翻译:

通过Achmatowicz重排,可以从糠醇中无氢解气相合成戊烷1,2,5-三醇
通过气相氢化衍生自糠醇的Achmatowicz中间体,已实现了戊烷1,2,5三醇(一种可生物再生的C5醇)的高效合成。在单组分或双组分Ni和/或Pt改性的介孔二氧化硅催化剂上进行氢化。该工艺的特点是不存在呋喃环的氢解反应,并具有100%的选择性,并具有其他绿色化学优点,例如在大气压下运行的温和且简单的无溶剂技术。双组分Ni / Pt改性介孔二氧化硅催化剂表现出最高的催化活性,其中10Ni1Pt / KIT-6最具活性,可提供高达100%的转化率。




















































 京公网安备 11010802027423号
京公网安备 11010802027423号