Nature Energy ( IF 49.7 ) Pub Date : 2019-09-09 , DOI: 10.1038/s41560-019-0450-y Yuvraj Y. Birdja , Elena Pérez-Gallent , Marta C. Figueiredo , Adrien J. Göttle , Federico Calle-Vallejo , Marc T. M. Koper
|
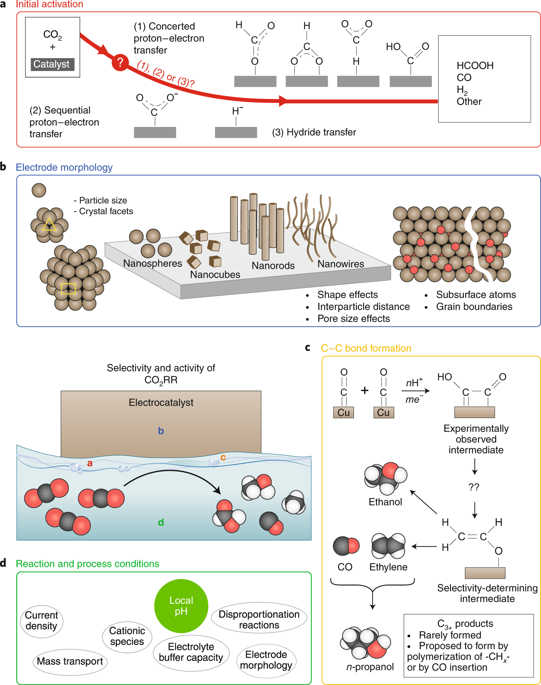
The electrocatalytic reduction of carbon dioxide is a promising approach for storing (excess) renewable electricity as chemical energy in fuels. Here, we review recent advances and challenges in the understanding of electrochemical CO2 reduction. We discuss existing models for the initial activation of CO2 on the electrocatalyst and their importance for understanding selectivity. Carbon–carbon bond formation is also a key mechanistic step in CO2 electroreduction to high-density and high-value fuels. We show that both the initial CO2 activation and C–C bond formation are influenced by an intricate interplay between surface structure (both on the nano- and on the mesoscale), electrolyte effects (pH, buffer strength, ion effects) and mass transport conditions. This complex interplay is currently still far from being completely understood. In addition, we discuss recent progress in in situ spectroscopic techniques and computational techniques for mechanistic work. Finally, we identify some challenges in furthering our understanding of these themes.
中文翻译:

在理解二氧化碳电催化转化为燃料方面的进展和挑战
二氧化碳的电催化还原是一种用于存储(过量)可再生电力作为燃料中化学能的有前途的方法。在这里,我们回顾了对电化学还原CO 2的理解的最新进展和挑战。我们讨论了CO 2在电催化剂上的初始活化的现有模型及其对理解选择性的重要性。碳-碳键的形成也是将CO 2电还原为高密度和高价值燃料的关键机械步骤。我们表明,初始CO 2活化和C–C键的形成受表面结构(在纳米尺度和中尺度),电解质效应(pH,缓冲液强度,离子效应)和传质条件之间复杂的相互作用的影响。目前,这种复杂的相互作用还远远没有被完全理解。此外,我们讨论了机械工作的原位光谱技术和计算技术的最新进展。最后,我们确定了在加深对这些主题的理解方面的挑战。

































 京公网安备 11010802027423号
京公网安备 11010802027423号