Separation and Purification Technology ( IF 8.1 ) Pub Date : 2019-09-09 , DOI: 10.1016/j.seppur.2019.116057 Boyuan Zhu , Hao Cheng , Yong Qin , Jianfeng Ma , Yong Kong , Sridhar Komarneni
|
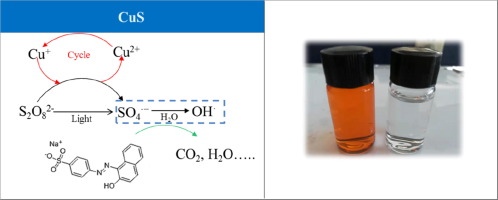
Copper sulfide (CuS) nanoparticles were prepared at 140 °C by a simple hydrothermal method and characterized by X-ray diffraction (XRD), scanning electron microscopy (SEM) and Brunauer, Emmett, Teller (BET) specific surface area method. Orange II (60 mg/L) was used as the target pollutant to investigate the degradation performance of CuS and K2S2O8 under visible light irradiation. The degradation efficiency reached 98.88% in CuS/K2S2O8/visible light system within 120 min, which showed very strong activity. Moreover, the catalyst also showed good stability and recycling performance for degrading Orange II. After three consecutive degradation cycles, the degradation efficiency of Orange II was found to be not less than 98%. The dye degradation mechanism was proposed as follows: Cu2+/Cu+ cycling catalyzed K2S2O8 to produce free radicals (SO4−), which significantly improved the oxidation ability of CuS/K2S2O8/visible light system.
中文翻译:

硫化铜与K 2 S 2 O 8一起用作出色的助催化剂,用于高级氧化过程中的染料分解
通过简单的水热法在140°C下制备硫化铜(CuS)纳米颗粒,并通过X射线衍射(XRD),扫描电子显微镜(SEM)和Brunauer,Emmett,Teller(BET)比表面积方法对其进行表征。以橙II(60 mg / L)为目标污染物,研究了CuS和K 2 S 2 O 8在可见光照射下的降解性能。CuS / K 2 S 2 O 8的降解效率达到98.88%/可见光系统在120分钟内显示出非常强的活动性。此外,该催化剂还显示出良好的稳定性和再循环性能,可降解Orange II。经过三个连续的降解循环后,发现Orange II的降解效率不低于98%。染料降解机理,提出了如下:铜2+ /铜+循环催化ķ 2小号2 ö 8产生自由基(SO 4 - ),其显著改善的CuS / K的氧化能力2小号2 ö 8 /可见光照明系统。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号