European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2018-03-02 , DOI: 10.1016/j.ejmech.2018.02.083 V. Hepnarova , J. Korabecny , L. Matouskova , P. Jost , L. Muckova , M. Hrabinova , N. Vykoukalova , M. Kerhartova , T. Kucera , R. Dolezal , E. Nepovimova , K. Spilovska , E. Mezeiova , N.L. Pham , D. Jun , F. Staud , D. Kaping , K. Kuca , O. Soukup
|
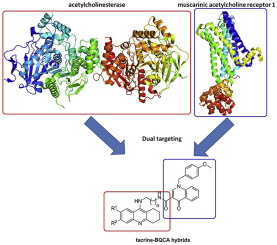
Novel tacrine-benzyl quinolone carboxylic acid (tacrine-BQCA) hybrids were designed based on multi-target directed ligands (MTLDs) paradigm, synthesized and evaluated in vitro as inhibitors of human acetylcholinesterase (hAChE) and human butyrylcholinesterase (hBChE). Tacrine moiety is represented herein as 7-methoxytacrine, 6-chlorotacrine or unsubstituted tacrine forming three different families of seven members, i.e. 21 compounds in overall. Introducing BQCA, a positive modulator of M1 muscarinic acetylcholine receptors (mAChRs), the action of novel compounds on M1 mAChRs was evaluated via Fluo-4 NW assay on the Chinese hamster ovarian (CHO-M1WT2) cell line. All the novel tacrine-BQCA hybrids were able to block the action of hAChE and hBChE in micromolar to nanomolar range. The hAChE kinetic profile of 5p was found to be mixed-type which is consistent with our docking experiments. Moreover, selected ligands were assessed for their potential hepatotoxicity on HepG2 cell line and presumable permeation through the blood-brain barrier by PAMPA assay. Expected agonistic profile towards M1 mAChRs delivered by BQCA moiety was not confirmed. From all the hybrids, 5o can be highlighted as non-selective cholinesterase inhibitor (hAChE IC50 = 74.5 nM; hBChE IC50 = 83.3 nM) with micromolar antagonistic activity towards M1 mAChR (IC50 = 4.23 μM). A non-selective pattern of cholinesterase inhibition is likely to be valuable during the onset as well as later stages of AD.




















































 京公网安备 11010802027423号
京公网安备 11010802027423号